Artificial
Insemination and Embryo Transfer
-
The advantages of AI and ET are numerous and include:
- an increased rate of genetic improvement;
- increased utilization of outstanding sires;
- increased utilization of outstanding dams with ET;
- better selection procedures;
- decreased risk of injury to humans, mares and stallions;
- decreased risk of disease;
- decreased risk of problems with overuse of a particular sire in an individual herd; e
- extending the usefulness of particular individuals in space (interstate and international shipping) and in time (semen is viable long after the demise of a sire or during times when a sire is unavailable for breeding);
- increasing the number of mares (and bitches to some extent) bred per day or with a single ejaculate.
- However there are also disadvantages.
-
Some disadvantages
include:
- use of AI requires estrus detection in the bovine,
- ovulation prediction in the canine and equine and ovulation detection in the equine;
- AI and ET are not allowed by all breeds in equine;
- ET requires synchronization of a recipient with the donor;
- they require trained personnel and some special equipment;
- there is a possibility of increasing the incidence of genetic abnormalities by overuse of a single sire;
- there is a decreased life span of frozen-thawed semen in the female tract compared to fresh semen
- A division of Monsanto ("Decisive"; http://getdecisive.com/asp/default.asp) now offers bovine sexed semen said to deliver the selected gender with 85% predictability
Semen processing
- In bulls, semen is usually collected with an artificial vagina (AV) rather than by electro-ejaculation (EEJ) because we can obtain a more physiologic sample and a more complete ejaculate.
- However, semen can be collected by EEJ if necessary.
- The frequency of collection will affect the number of sperm / ejaculate and therefore the number of inseminates / ejaculate.
- Frequency will vary with the species and will be covered in other lectures.
-
For
more information on semen collection and evaluation, see the BSE notes.
Fresh, cooled semen
- This is used primarily in the equine and canine.
- In general, after collection and evaluation, semen extender is added to the ejaculate.
-
Various semen extender recipes are available but in general they:
- provide nutrients for the sperm during storage,
- protect against cold shock,
- contain antibiotics to inhibit bacterial growth,
- buffer the extended semen against changes in pH,
- maintain proper osmotic pressure,
- increase the volume without decreasing viability thereby making the semen easier to work with, and
- protect the sperm cells during the freezing and thawing process.
- Semen extender can be prepared according to a
"recipe" or purchased commercially.
- Numerous products available for use with various species for either fresh cooled semen or frozen semen.
- Most extenders for fresh cooled use are nonfat dry skim milk based. Most extenders for freezing contain egg yolk.
- Osmolarity and pH of the extender is very important so unless you are using a lot and can save significant money, you are probably better off to purchase a commercial product.
-
Sperm will quickly metabolize available substrates in seminal
plasma so motility will decrease rapidly. Therefore, after the semen is
collected, it should be mixed with extender as soon as possible, preferably
within 10 or 15 min.
- For stallions, the ratio of semen to extender should be > 1:2, and a ratio of 1:4 or 1:5 is preferable.
- Nevertheless, it is the final concentration of sperm in the extended sample that is the critical factor.
- Longevity of the sperm cells is maximized if extender is added to give a final concentration of sperm cells of 25 - 50 million/ml.
- With an adequate extender at a proper dilution, sperm will retain their fertilizing capability for up to 24 hours at room temperature (20oC), depending on the individual stallion.
-
Therefore a semen: extender dilution factor should be calculated based on the
initial concentration of sperm to give a final concentration of 25 - 50 million
sperm/ml before shipping.
For example:Concentration at collection = 267 million/ml needed therefore, use 5 parts extender : 1 part semen to give a final extended concentration of 45 million sperm / ml
Desired concentration = 50 million/ml;
267 / 50 = 5.3 total parts semen + extender
5.3 total parts - 1 part semen = 4.3 parts extender
-
If a stallion provides an ejaculate with a low concentration,
so that dilution at a 1:4 ratio, for example, would result in the final
concentration being less than 25 million/ml, centrifugation is recommended.
- Centrifugation can be used to concentrate the semen so that dilution at a recommended ratio can be achieved while maintaining a concentration of 25 - 50 million/ml.
- Recommendations for centrifugation are to begin with a force of 500 X g for 10 minutes.
- Less time or fewer g's will result in less damage to the sperm from the force of centrifugation but a softer pellet and more viable motile sperm in the supernatant that will be discarded.
- Centrifugation for a longer time or at higher g's will achieve a better recovery and minimize sperm losses in the supernatant but result in more damage to the sperm cells.
- Centrifugation technique can be altered within a range of g's and times to achieve good recovery while minimizing cellular damage.
- After centrifugation, the supernatant is removed and discarded.
- Studies have shown that a small portion (a minimum of 5%) of the seminal plasma must be left with the sperm to preserve viability.
- The pellet is then re-suspended using sufficient extender to achieve a final concentration of 25 - 50 million/ml.
- Preservation of semen quality depends to a large extent on the initial quality of the semen, and varies from stallion to stallion.
-
Little work has been done with canine to determine the
optimum concentration but a 1:1 ratio (semen: extender) has been used
successfully.
Determination of insemination dose
- The recommended insemination dose is usually 500 million progressively motile (normal) sperm, although acceptable pregnancy rates can be achieved with as few as 250 million normal, progressively motile sperm.
- The volume of the inseminate is not critical.
- Although pregnancy can be achieved with very small volumes, the recommended minimum volume used for insemination is usually 10 ml.
- Usual insemination doses with fresh cooled semen range from 20 to 120 ml.
- Although some veterinarians are reluctant to inseminate volumes greater than 60 ml, studies have shown no decrease in fertility when larger volumes were inseminated, provided the inseminate was not real dilute.
-
To determine the volume of semen to package for shipment,
divide the desired number of progressively motile sperm (usually 500 million) in
an insemination dose by the product of the concentration times percent motility
at the end of the storage period.
- For example, if we have extended our semen to a concentration of 50 million/ml and our motility after transport is 40%, we should package at least : 500 / (50 X .40) = 500 / 20 = 25 ml.
- Unless a stallion is in very high demand it is best to package more than the minimum amount needed.
- For example, with the above stallion, packaging 50 ml would insure that more than adequate numbers of sperm were available for fertilization of the oocyte.
- Furthermore, a more conservative method is to include percent normal morphology into the equation.
-
For the stallion above, if normal morphology is
70%, the equation becomes:
500 / (50 X .40 X .70) = 500 / 14 = 35 ml
Packaging
-
Various brands of shipping containers are available.
-
The Equatinter
system is one of the first containers that became available and has
remained popular.
- It is reusable, durable and does a very good job of maintaining the semen viability.
-
Numerous "disposable" brands are on
the market including the
- Lane STS,
- Bioflite,
- Equine Express,
- disposable Equitainer.
- Expect-a-Foal, and others.
- Most do a decent job of maintaining viability.
- Some do just as well as the Equitainer.
- The advantage is that because of the low cost, the price of the container can be added to the cost of the semen shipment.
- Most of the "disposable" containers can actually be re-used.
-
The Equatinter
system is one of the first containers that became available and has
remained popular.
-
Sperm remains viable for 24 to 48 or more (up to 72 with some
containers) hrs.
- Data on the various containers comparing rates of cooling, lowest temperature reached, etc. is available.
-
Ideally, the semen should be extended and placed in the
cooling device within 10 minutes of collection.
- The rate at which extended semen is cooled is critical.
- If the cooling rate is too fast or too slow, sperm viability is decreased.
- A cooling rate of -0.05 to -0.1 C/min is desired between 20 and 5 C.
- Ideal storage temperature is 4-6 C.
- Basically, all the containers are an insulated packaging system with an "ice pack".
- The pre-warmed extender should be added slowly to the semen.
- The extended semen should be placed in a container such as a Whirl-Pak bag, from which air can be excluded, and sealed.
- This container should then be placed in a second container from which air is again excluded and the second container also sealed.
- The extended semen is placed in the container and the container assembled according to the instructions.
-
Information should be included with every shipment identifying the
semen donor and characteristics of the semen shipped (motility, concentration,
etc.) at a minimum.
- An information form should be included with each shipment. Minimum information required on the form includes stallion identification, date of collection, concentration and volume of inseminate shipped (i.e. numbers of sperm), initial motility, type of extender used, numbers of doses shipped and any special instructions.
- The semen is then shipped overnight or same day to its destination.
-
When the semen is received, a quick evaluation of the motility (of
a warmed sample) is made.
- The semen is then inseminated into the mare (or bitch) without any pre-warming or addition of other substances.
-
It has become commonplace for some people to place two
insemination doses in the shipping container, intending one to be used upon
arrival and the other to be used the following day.
- In fact, there is no physiological basis for such practices.
- The ideal place to store spermatozoa is in the mare's oviducts.
- Man-made semen transport devices are a means to transport semen from the stallion to the mare without transporting horses.
- They are not meant to take the place of the mare as a site of sperm storage until fertilization.
- All semen received in a transport device should be inseminated into the mare at the time of arrival. This should be kept in mind when preparing the semen for shipment.
-
Some reports indicate sperm viability is decreased due to
contact with the rubber plunger on syringes.
- Syringes constructed of all plastic are therefore recommended by some researchers. In actual practice, however, the extended semen is not in contact with the plunger very long and the numbers of sperm cells being inseminated are great enough that toxicity from the syringe is probably of little clinical significance.
-
If semen is held in syringes for any
length of time, however, as in some types of shipping devices (Salsbro box),
syringes of all plastic should be used.
Transport and Insemination
-
After packaging, the container is shipped by commercial
carrier or airline to be delivered within 24 hours.
-
Some concerns have been
raised about x-radiation of semen as it passes through airport security.
- Studies examining the effects of doses of radiation used in airport security have found no adverse effects on spermatozoa.
- However, there are indications that the airports will soon increase the level of radiation in an attempt to improve security and the effect of the increased level of radiation is unknown.
- The Equatinter system has a lead shield in the transport container to shield the semen from the radiation and any possible harmful effects.
-
Some concerns have been
raised about x-radiation of semen as it passes through airport security.
-
When the semen arrives at its destination, the mare is
prepared for artificial insemination.
- Either while the mare is being prepared or after she is inseminated, the semen should be examined to determine percent motility.
- Care must be taken to maintain the semen at the chilled temperature in the container until ready to place it into the mare.
- The best place to pre-warm the semen is in the mare's uterus.
- Pre-warming the semen before placing it into the mare decreases conception rate.
- A drop may be removed from the sample container and placed on a warm microscope slide on a slide warmer.
- A warm cover slip is placed on top and motility estimated in the same manner as during a breeding soundness examination.
- Motility will improve as the sample is allowed to warm.
-
The concentration may also be determined if it is unclear how many
intended insemination doses were sent.
Frozen Semen
- Frozen semen can be stored indefinitely at -196o C in liquid nitrogen.
- This is more convenient to use in some regards because shipping can be done well in advance of the need for semen.
- Semen can be used long after a sire is dead.
- In species with a short estrus and predictable ovulation (e.g. bovine) the farmer can have a tank of semen on the farm to use as cows come into estrus.
- Frozen semen allows
banking of semen, for example semen quality is highest in bulls not old enough
to complete progeny testing.
- Progeny testing in cattle provides a means to use superior sires and to test for genetic defects.
- However, the sperm of some individuals doesn't freeze well and some sperm are lost (die) in the processing and freezing.
- In species with a longer estrus or less predictable time of ovulation (e.g. mare, bitch) frozen semen is not quite as convenient due to the reduced longevity of the sperm after thawing.
- Frozen - thawed semen may be partially capacitated, thereby explaining the reduced life span and requires insemination closer to ovulation for good fertility.
- With frozen equine semen, mares are either palpated/ultra sounded every 6 h and inseminated as soon as ovulation is detected or palpated/ultra sounded twice daily (sometimes more) and inseminated when ovulation is deemed imminent.
- The protocol used depends on the veterinarian's preference and on the availability of semen (i.e. if semen is limited , such as with a dead stallion or very expensive semen, post ovulation insemination is more common).
- Therefore use of frozen semen in horses requires
very good management to achieve acceptable fertility
Storage of frozen semen (& embryos)
-
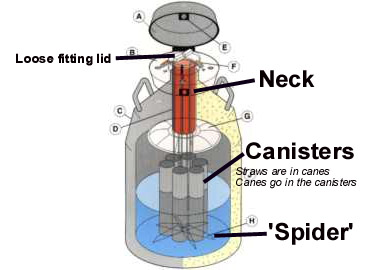
Tank construction:
- Liquid nitrogen tanks are constructed of two tanks, one inside the other, connected at the top or "neck."
- The space between the two tanks is packed with insulation and a vacuum is created.
- The neck of the tank is its weakest point and can be easily damaged, breaking the vacuum in the tank and causing it to lose its ability to maintain an extremely low temperature.
- Frost on the outside of the tank, near the neck, indicates damage to the tank and loss of its insulating properties.
- Objects should not be stored on top of the tank, nor should it be used as a seat.
- Skidding the tank across the floor or banging it against the wall or floor can similarly damage the neck tube.
- Scratches or dents in the outer tank can lead to vacuum loss and reduced holding time.
- The nitrogen in the tank will need to be replenished and a service contract can be arranged for periodic refills of nitrogen.
-
The level of nitrogen in the tank should be monitored on a regular
basis, monthly or weekly, depending on the style and size of the tank and the
frequency of use.
- The level of nitrogen is checked by placing an instrument such as a yardstick into the tank with the tip resting on the bottom, for a few moments.
- The stick is then removed and waved in the air, allowing frost to form on the stick to reveal the level of nitrogen.
- A record should be kept, noting how much nitrogen is in the tank each time it is checked.
- This will serve not only to encourage a regular schedule of monitoring the tank but also will provide warning if the tank is beginning to fail.
-
All tanks will eventually fail
but usually it will be a slow, gradual failure.
- Special monitor ampoules are available which will show if the temperature within the tank has risen to a dangerous level.
- The frequency with which the nitrogen will need to be replenished depends on the particular tank and the frequency of use.
- A semen inventory should be maintained so that straws of semen can be found quickly and easily.
- When handling semen, the canister should never be lifted higher than 1¼ in. below the top of the neck tube. Semen raised higher than this level will undergo an increase in temperature that will result in a decrease in quality.
-
Liquid nitrogen tanks usually contain 6 canisters.
- Each canister holds a number of canes.
- On each cane are 2 goblets, one above the other. Each goblet contains a number (usually 5 or 10) of straws.
-
Straws are
either 0.5 or 0.25 ml.
- Equine semen is also packaged in 5 ml (or 2.5 ml) maxi-tubes, which are stored directly in the canister, without canes or goblets.
- Other packaging systems include aluminum pouches or pellets.
- Typically, straws are labeled with information which identifies the semen donor, processor, date, batch, etc.
- Bovine semen is usually processed according to standards set up by the National Association of Animal Breeders (NAAB) and Certified Semen Services (CSS).
- AKC has requirements for the information that must be included on straws of canine semen registered with AKC.
-
No standards are currently in place for equine semen.
-
Semen handling
- Semen stored in liquid nitrogen at -196o C will last indefinitely.
- However, repeated warming, even though still frozen, is detrimental.
- There is a temperature gradient at the neck of the tank from -196 C to room temperature. Repeated exposure at the neck of the tank will reduce semen viability post-thaw.
- Therefore semen should not be raised into the neck unless being removed from the tank. Semen should be kept as low as possible in the tank.
- Higher goblets are at more risk than lower goblets if the tank is not maintained and semen handled properly.
- Rule of thumb for thawing French straws is to thaw in a 95 F water bath for 30 - 60 sec. HOWEVER, semen should always be thawed according to the directions provided.
-
There are variations in thawing technique and/or
temperature for various freezing protocols and packaging systems.
Insemination
-
AI in cattle is done by threading the insemination pipette
through the cervix per rectum.
- Semen should be deposited within the uterus.
- Because frozen semen does not traverse the cervix very well, placement of semen in the cervix will reduce chances of conception.
- Basically, the cervix is located per rectum, the vulva is cleaned and the AI gun inserted.
- The AI gun is then threaded through the cervix.
-
The dogma has been that semen should be
deposited in the uterus, just inside the internal cervical so.
- However, recent work has shown that farther up into the uterus may be a more "identifiable" location and that inseminators may have more success when using the uterine horn as the target.
- No detrimental effect has been observed by placing the semen further into the uterus. Numerous studies have shown the ability of semen to migrate from one horn to the other, so side of deposition in relation to the follicle is unimportant.
-
AI in mares is done as a sterile procedure via a vaginal
approach.
- Semen is deposited in the uterus, after the pipette is passed through the cervix.
- The major problem with the use of frozen semen in mares is the timing of insemination.
- Frozen semen does not have a long life-span after thawing.
- Therefore, insemination must be timed closely to ovulation.
- Because of the long and variable estrus in the mare and the fact that she ovulates during estrus, frequent monitoring is necessary for success when breeding with frozen semen.
- If semen is plentiful and relatively inexpensive, insemination can be done before ovulation, when ovulation is predicted to occur within the next 12 - 18 hr.
- More often, semen is in limited supply and is expensive, In that case, the mare is palpated and ultra sounded every 6 hr and is inseminated as soon as ovulation is detected.
-
In this way, the semen is placed in the mare within 6 h
of ovulation and the oocyte is still viable.
Evaluation of frozen semen
-
Bovine (see Soc. Theriogenology fact sheet)
- Equine
- Many tests have been examined in an effort to judge the fertility of frozen-thawed semen. However, to date no test has been shown to correlate highly with fertility.
- For this reason, motility 15 min post thaw is usually used as an estimate of semen quality.
- Unfortunately, there are many stories of semen with good post-thaw motility never achieving a pregnancy and semen with poor motility having acceptable conception rates.
-
Clearly, there is
much to be learned.
Stages in embryo collection and transfer
Synchronization of the donor and recipient
- Synchronization of the donor and recipient is done to synchronize not only the circulating progesterone concentration, but to synchronize the degree of eudiometrical development between donor and recipient.
- This is important in establishing maternal recognition of pregnancy.
- Bovine estrus synchronization has been covered previously.
-
In mares, ovulation
synchronization, rather than estrus synchronization is the goal.
-
Numerous
schemes exist, including
- multiple PGF injections at 14-15 d intervals, with estrus expected within 6d of the 2nd injection;
- altrenogest or progesterone treatment for 8 d with PGF on the last day, with estrus expected within approx. 4 d; and
- a combination of progesterone (150 mg) and estradiol (10 mg) IM, daily, for 10 d with PGF on the last day.
- The progesterone and estrogen combination provides the best synchrony of ovulation.
- With any of these plans, hCG is used to induce ovulation.
-
Recent reports indicate altrenogest is effective in
delaying ovulation but has the side effect of
-
Must identify day of ovulation (frequent palpations,
ultrasound)
Super-ovulation of the donor animal
-
Numerous
schemes exist, including
-
In ruminants, many different hormones and programs have been
used to stimulate the donor animal to produce more than one oocyte that can be
fertilized.
-
These include:
- FSH-P (withdrawn from market);
- purified FSH products such as Super-Ov,
- Boltrope,
- Ovagen, etc;
- equine FSH;
- eCG; and
- anti-Inhibin.
-
Hormones are usually injected over a period of 4-5 d, once or twice daily; often
started after 7-9 d of progesterone suppression.
-
Example of typical super-ovulation scheme:
Day 0 Estrus
Day 10 Inject 5 mg FSH every 12 hr
Day 11 Inject 4 mg FSH every 12 hr
Day 12 Inject 3 mg FSH every 12 hr
Day 13 Inject 2 mg FSH every 12 hr; inject PGF with p.m. injection
Day 14 Inject 1 mg FSH every 12 hr
-
In mares, no practical method is available to give good
results, experimental work with anti-Inhibin, pituitary extracts, etc. has
increased the rate of double ovulations but has not resulted in true
super-ovulation.
Fertilization of the donor animal
-
These include:
-
Obviously, insemination must be timed to ovulation. This
requires heat detection in cattle and prediction of ovulation in mares. (See
above)
Collection of embryos
- In both cows and mares, this is usually performed 7 days after ovulation.
-
In cattle, embryos are found in the tip of the uterine horns
when collected at 6-8 d after estrus.
- Collections are usually performed transcervically, one horn at a time.
- Alternatively, embryos may be collected laparoscopically or surgically.
-
In mares, embryos are collected at d 6-9 after
ovulation.
- Recovery rates are best at d 7-9 but transfer of d 9 embryos results in reduced pregnancy rates.
- Therefore recovery is usually performed at 7 d.
- In contrast to cows, the entire uterus is flushed rather than one horn at a time.
- This is due in part to anatomical differences and because the embryo migrates throughout the uterus.
- Embryo collection is usually via a Tran cervical approach per vaginum.
-
Flushing medium such as phosphate buffered saline (PBS) with 1%
serum and antibiotics is infused into uterus to bring the embryo's) into
suspension and then recovered.
Isolation, evaluation and transfer of the embryos
- The collected medium with the embryo's in it is filtered and then examined under a stereo microscope and the embryo's identified.
- The embryo's are evaluated under higher magnification, washed and cleaned by transferring to successive dishes of transfer medium.
- Transfer medium usually contains 10% serum in addition to the PBS.
- The embryo can then be transferred fresh to a synchronized recipient, either surgically or non-surgically.
-
The
embryo is placed intrauterine,
- in ruminants it is placed ipsilateral to the CL (which is identified before transfer),
- in mares it is simply placed in the uterus.
- During the transfer procedure, attempts are made to minimize any PGF release.
-
Success rates with non-surgical transfer (by an experienced person) are
very similar to surgical.
