Equine
Abortion

179-194
Infectious Abortion Equine
herpesvirus -1 (EHV-1, EHV 11, Rhinopneumonitis)
- EHV-1 causes respiratory infection in all ages,
however the disease is more severe in younger animals. It also
causes abortion and neurologic disease. (CNS signs are due to
thrombotic lesions, not infection of neural tissue) EHV-4, also called
EHV-12,
causes respiratory disease, only rarely neurologic or abortion
disease. They are now considered to be 2 separate viruses (fam.
Alphherpesvirinae, gen. Varicellovirus)
-
Primary replication is in the mucosal
epithelium, then a lymphocyte associated viremia develops
-
The virus persists in the
pregnant mare for long periods with the majority of abortions (90%)
occurring within 60 d post-infection; however, the range from
infection to abortion is 14 to120 days.
-
Abortions
may occur from the 5th month to term, but most commonly occur from 8
to 9 months to term.
- Infection of the fetus occurs by
migrating leukocytes and/or infection of umbilical blood
vessels; primary target organs are the respiratory
tract and liver.
- Premonitory signs of abortion are few.
- Typically there is a sudden abortion without milk or udder
development.
- The fetus is delivered in a fresh condition, and may
even be born alive and die soon after.
- Immunity is short after
natural infection (3-4 mo) and individuals may be reinfected
repeatedly.
- Transmission is by the respiratory route.
- Most mares of breeding age are clinically
immune to respiratory disease but not to abortigenic infection.
- Spread of EHV-1 through a herd occurs most
commonly without clinical signs.
- The virus can persist in the trigeminal ganglia
and can recrudesce in latent carriers. Stressing pregnant mares may
trigger abortion.
- Diagnosis of abortion is by histopathology of
the fetus.
- Fetal lesions include serous pleural and peritoneal
fluid, hemorrhages of the entire respiratory tract, petechia of the
oral mucosa and conjunctiva, focal hepatic necrosis with
intranuclear
inclusion bodies in liver and lung*, and a necrotic and friable
thymus.
- Specimens for submission to the diagnostic lab should
include lung, liver and thymus, both frozen and in formalin.
- Serology of the mare is of no value because of the time period which
has lapsed between maternal infection or viremia and abortion.
Diagnosis may also be confirmed by virus isolation or PCR.
- Control is achieved through vaccination
programs.
- Both modified live and killed vaccines are available.
- Controlled studies have showed that both are effective, both had
failures.
- Recommendations are to vaccinate pregnant mares with 3
doses, given at 5, 7 and 9 mos. of gestation.
- (Note: in addition,
pregnant mares should be given their annual booster against flu,
tetanus, encephalitis, etc. at 10 mos gestation which serves not
only to protect them for the coming year and gives them their
tetanus booster which is important in case of any postpartum
complications but also boosts immunoglobulins at a time when
colostrum is being produced so as to provide better protection for
the neonate)
- Non-vaccination programs are practiced in some
countries. A lower incidence of disease is reported in countries
where vaccination is not practiced, however, the disease is common
enough in the US that not vaccinating pregnant mares is inviting
disaster.
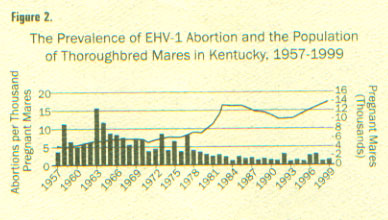 
(from ML Vickers, DG Powell, Equine Disease Quarterly
Newsletter, Dept Vet Sci, Gluck Eq Res Center, Univ KY; )
Equine viral arteritis
- The disease was unknown before 1953. In 1953
the virus was isolated, the etiology defined, disease described,
etc.
- Studies from which the descriptions of the
disease are derived are generally based on an experimentally
derived, highly virulent strain (Bucyrus strain) that was made by
serial passage and became VERY virulent.
- Some outbreaks of the disease such as the 1984
outbreak in Kentucky during the breeding season, are not associated
with abortions. Other outbreaks are associated with significant
pregnancy losses.
- Naturally occuring abortion from EVA is not
common. It occurs sporadically, sometimes in clusters.
- Majority of abortions occur 23-57 days following exposure, or
6-29 days following the onset of fever in the mare.
- Foals can be born infected and die within 72 hr
(appear normal when born or may be born ill, develop respiratory
signs or enteritis)
- In a controlled study, mares were bred to
carrier stallions, then transported and subsequently housed with
mares that were in the 7-11th month of gestation.
- All mares became
seropositive.
- Clinical signs would have gone unobserved but for the
experimental protocol which included BID temperatures and weighing
of feed given and remaining at the end of the day.
- A slight febrile
response and mild anorexia was detected.
- Of the exposed mares, 10 of
14 pregnant mares aborted. In this study, autolytic changes were not
observed in the fetuses.
- The virus was isolated from the fetus or
the placenta in 8 cases.
- Some breeds (e.g. Standardbreds) have a high
incidence of seropositive individuals and disease is almost never
seen.
- Other breeds are quite naive and clinical signs
(i.e. abortion) are more common.
- There may be some differences in strains of the
virus.
- Typically there are no clinical signs in the
mare other than abortion.
- After abortion, fetal tissues to collect for
laboratory diagnosis are: lung, liver, placenta, fetal fluids - if
EAV is the cause of the abortion, it is fairly easy to isolate.
- Transmission is by oronasal and venereal
routes. Respiratory shed occurs for about 1.5 - 2 wks
- If a stallion develops clinical signs, control
the fever and scrotal edema to prevent temporary sterility
- After infection, mares clear the infection and
develop immunity.
- However, the virus is shed in the semen and the
majority of infectious organisms are in the seminal plasma.
-
Stallions then become inapparent carriers and remain so for years.
- Need to keep about 90 yds between a shedding
stallion and other horses; or between a mare bred to a shedding
stallion and other mares
- The disease can be transmitted by AI and has
been transmitted by fresh, cooled semen. As the use of transported
(fresh cooled or frozen) semen has become more common and
international trade in equine semen has increased, reports of
abortion due to EVA have become more common as well.
- Control is by use of a MLV vaccine. Once
vaccinated, a stallion cannot be distinguished from a naturally
infected animal by serology, therefore there is some reluctance
towards widespread use of the vaccine. However, virus isolation from
the semen can distinguish carriers from vaccinates.
Bacterial abortion
- There are 3 proposed routes of infection.
- The
first involves the hypothesis that bacterial levels in the uterus
can be low enough to allow conception, but multiply during gestation
producing placentitis and abortion. The other routes are more
plausible.
- The second involves hematogenous spread of a pathogen.
-
The third and most common route involves transcervical migration of
the pathogen.
- Etiological agents involved can be almost
anything.
- Staph, Strep, E coli are commonly implicated and a whole
range of organisms have been reported.
- Normally there are
premonitory signs of an impending abortion, such as udder
enlargement, mammary secretions and relaxation of the vulva.
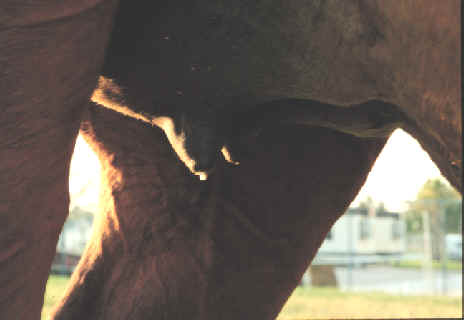
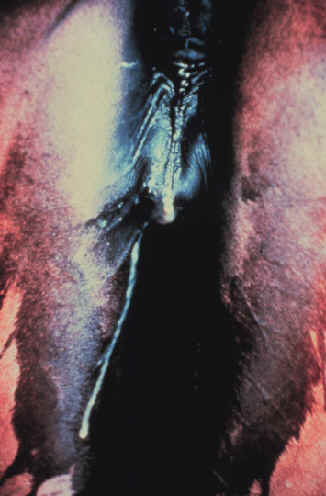
- Autolysis of the fetus and placenta can make
identification of the etiologic agent difficult.
- After an abortion,
the chorionic surface of the placenta should be inspected.
- If the
infection is an ascending infection through the cervix, a thickened
area extending from the cervical star will be evident.
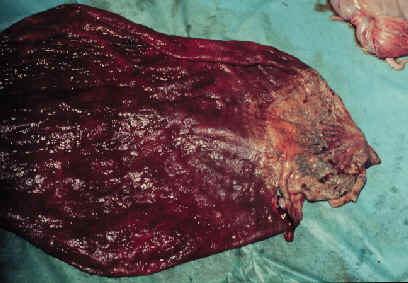
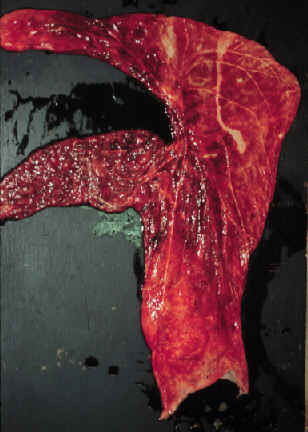
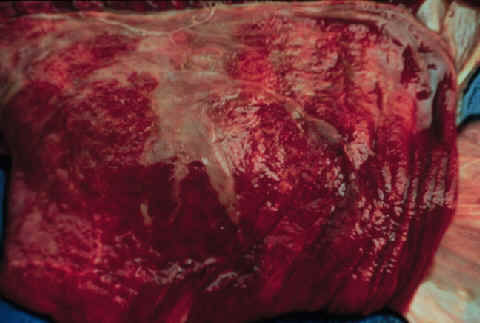
- The affected
portion of the placenta will be thickened, discolored and the villi
will be blunted. It may be covered with a brownish, tenacious
exudate.
-
Pre-partum placental inspection
-
Placentitis is a leading cause of abortion and neonatal disease in
horses. Early diagnosis is critical to be able to begin effective
treatment that can result in the survival of a healthy foal.
-
Placentitis
is initiated either through the hematogenous spread of bacteria to the
chorioallantois or through a transcervical route by bacteria ascending
through the cervix
-
The ability to diagnose placentitis in utero, before clinical signs of
premature lactation or vaginal discharge are observed will improve the
chances of successful treatment.
-
Although it cannot be recommended to
examine all mares routinely at intervals for placental abnormalities,
mares with a history of problems or at high risk may be periodically
examined for signs of placental disease.
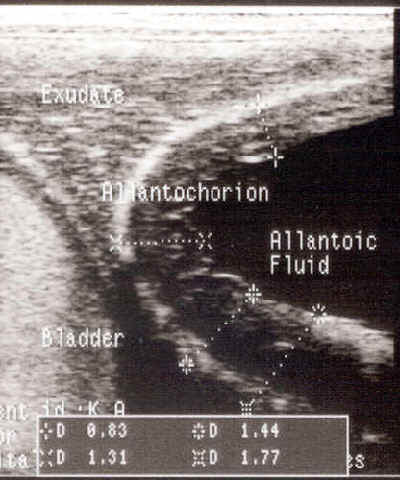
-
Ultrasonographic examination of
the placenta may be done either transabdominally or per rectum.
- Observance of thickening of the uteroplacental
unit, separation between the uterus and placenta or exudate between
the uterus and placenta are indications of a problem. Because the
area of the cervical star is most commonly affected, ultrasound exam
of that area per rectum is useful.
-
Reef et al., have described the assessment of fetal well being in utero
using a transcutaneous approach.
-
The uterus is examined at multiple
sites, attempting to cover all four quadrants.
-
Fetal heart rate, the
diameter of the fetal aorta, fetal activity, maximum depth of fetal
fluids, utero placental contact and utero placental thickness have been
found to be related to the pregnancy outcome.
-
Significant utero
placental thickening is consistent with placentitis, while anechoic
spaces between the uterus and placenta suggest placental separation.
- While observed abnormalities are good indicators of placental disease,
the lack of abnormal signs on transcutaneous examination does not mean
that placental disease does not exist.
- Focal areas of placentitis may go
undetected.
-
Most cases of placentitis stem from the spread of bacteria through the
cervix, infecting the chorioallantois in the region of the cervical star
and spreading from there.
-
Troedsson et al., have examined the utero
placental unit near the cervical os per rectum, near the area where most
cases of placentitis would be expected to originate.
-
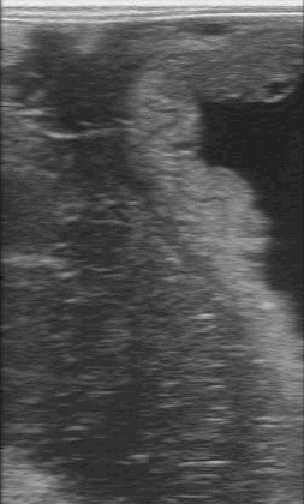
Measurements of the
combined thickness of the uterus and placenta at the ventral portion of
the uterus, near the cervical os, are obtained.
-
The normal thickness of
the utero placental unit is dependent on gestational age (8 mm at 270 to
300 days gestation, 10 mm at 300 to 330 days, 12 mm at greater than 330
days).
-
Multiple measurements should be taken and the mean value obtained
because a single value may be misleading, especially if the image is
taken at an oblique angle.
-
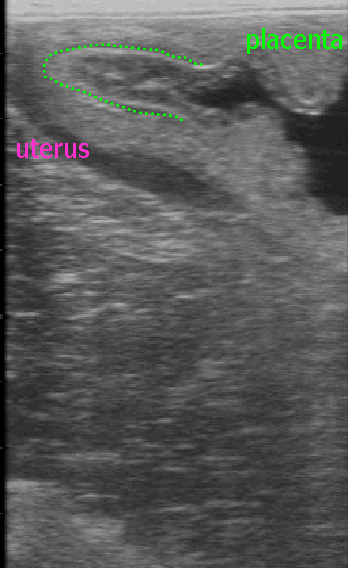
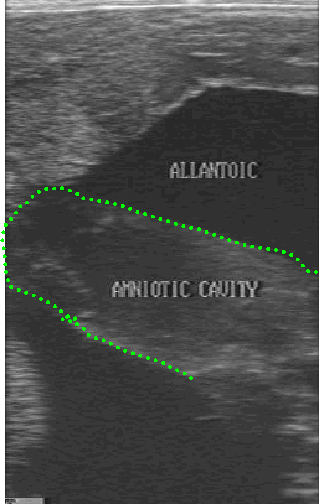
-
Pre-partum treatment
- If placentitis is diagnosed in utero, treatment should be initiated
immediately.
- If a vaginal discharge is present a culture and sensitivity
may be beneficial in choosing an appropriate antibiotic.
- While results
of the culture are pending or if no discharge is present, broad spectrum
antibiotics should be administered.
- Research has shown that in normal
pregnancies, without placental disease, that sulfa-trimethoprim can
cross the placenta better than penicillin or gentamicin.
- However, no
studies have been reported examining the ability of various antibiotics
to achieve significant levels in the chorioallantois and fetal fluids in
cases of placental disease.
- Nevertheless, sulfa-trimethoprim is often
chosen to begin treatment, not only because of these studies but because
of the expected duration of therapy and the ease of oral administration.
- Treatment also includes progestogen supplementation and pentoxifylline
to aid in preventing pregnancy loss.
- Depending on the nature of the
problem, endotoxin release may be associated with placental disease.
- Endotoxin can result in endogenous prostaglandin release leading to
abortion or premature delivery.
- Exogenous progesterone or progestogen
supplementation has been shown by Daels et al., to be effective in
preventing abortion associated with prostaglandin release.
- The oral
progestogen, altrenogest (0.88 mg/kg), is usually used not only because
of ease of administration, but because work by Daels et al., has shown
it may be more effective than injectable progesterone.
- In addition to
antibiotics and progestogen supplementation, pentoxifylline (4 gm, p.o.,
BID) has been recommended (Byars) for its anti-endotoxic effects in
cases of placentitis.
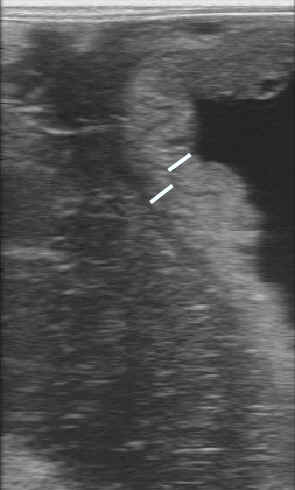 

- If evidence of placentitis exists, treatment
consists of: altrenogest (0.088 mg/kg, PO, SID; =1 ml/25 kg = double
dose), trimethoprim sulfa (30 mg/kg, PO, BID), pentoxifylline (4 gm,
PO, BID)
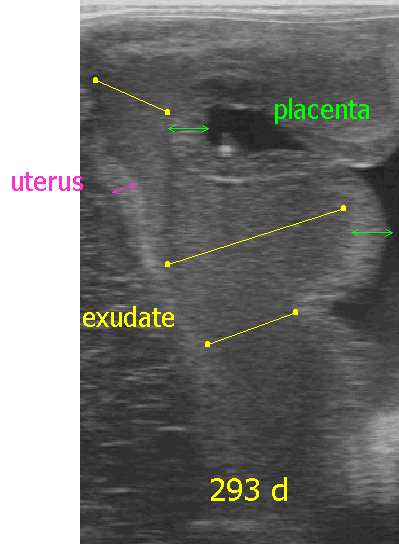
- A specific cause of placentitis, found primarily in KY and the surrounding area, has been identified as a nocardioform organism, Crossiella equi. This disease is characterized by an area of placentitis with a tan mucoid exudate located at the base of a uterine horn, near the body of the placenta. It has been identified in other locations (e.g. Florida). It occurs sporadically and in 1998 and 1999 was the most common cause of placentitis in KY.
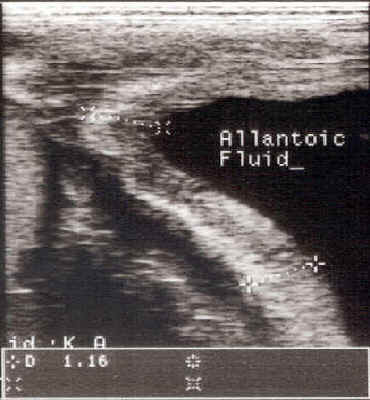
Mycotic abortion
- Abortions result from a placentitis due to
fungi, especially Aspergillus
sp.
Infection is thought to originate from the respiratory tract,
through the circulatory system to the uterus and placenta.
Alternatively, the transcervical route is also possible. There is a
higher incidence in stabled mares.
- Typical gross lesions of placenta include
necrotic areas that are difficult to distinguish from bacterial
lesions. The chorionic surface may be dry, thick, and leathery.
There may be lesions on the fetus, especially the skin. Fungal
elements are easily demonstrable in smears and cultures if they are
responsible for the abortion.
Leptospirosis
- Leptospirosis is being increasingly identified
as a cause of abortion. Incidence varies greatly from year to year.
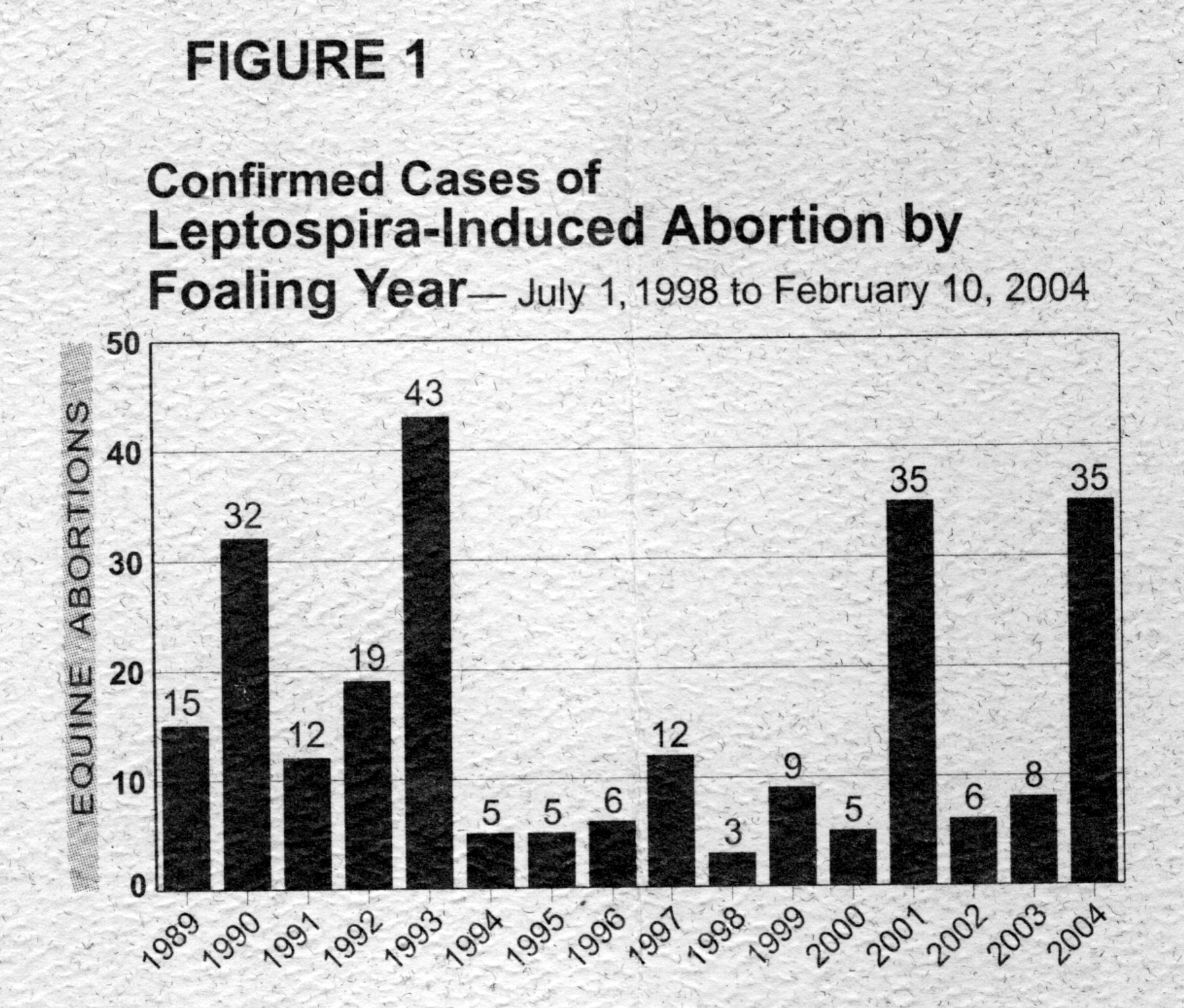
(from M Donahue, B Smith, Equine Disease Quarterly
Newsletter, Dept Vet Sci, Gluck Eq Res Center, Univ KY; )
- Leptospira interrogans serovar
pomona had been reported to be most often implicated in
equine abortion. However, in the Equine Disease Quarterly Newsletter (Dept. Vet. Sci., Gluck Eq. Res. Center, Univ KY), Drs. M Donahue and B Smith reported of 250 cases diagnosed over the last 16 years (through the 2004 foaling season) 210 (84%) were due to kennewicki and 24 (10%) to grippotyphosa. The raccoon is the maintenance host for grippotyphosa but the maintenance host for kennewicki was unknown at the time of the report.
- L. bratislava ,
thought to be "host adapted" in horses may be implicated
in some disease processes.
- Many of the general points of information
regarding lepto infection in cattle are applicable to horses.
Bacteremia occurs after a 4-10 d incubation; pathogenic serovars
localize in the kidneys or genital tract; favorable environmental
conditions are moist, warm situations; invasion occurs through
mucous membranes, soft moist skin, etc.
- Clinical signs in the mare are often
unobserved.
- Abortions occur from 6 mos to term. Gross
lesions of the fetus vary and may include icterus and a swollen
liver and/or kidneys.
- Placental lesions are common.
- Diagnosis is by identification of the organisms
and by serology of the mare.
- No vaccine is currently available.
-
Control is achieved by sanitation and hygiene.
- Some reports indicate
potassium penicillin may be useful in pregnant mares with rising
titers. Human studies indicate penicillin, IV, in high doses, may be
a reasonable treatment.
Salmonella
aborttus
equi
- This organism causes abortion in mares,
septicemia in foals and testicular lesions in stallions. The
incidence has decreased to near non-existence but is reported in
some areas of the world. Transmission is by ingestion or possibly
venereal. Diagnosis is by recovery of the organism. Control is by
isolation and hygiene and a bacterin has been available.
Non-infectious
abortion Twinning
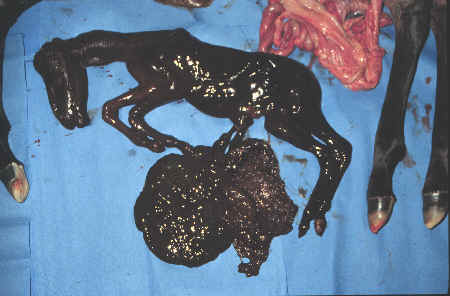
- The usual outcome of twins is abortion,
stillbirth, mummification or dystocia.
- It has been considered to be the most important
cause of abortion, with EHV-I under control. However, with the
advent of early pregnancy diagnosis with ultrasound, the incidence
has decreased greatly.
- Multiple ovulations are common, up to 35% in
some breeds. However, twins are not common.
- The difference between the double ovulation
rate and the lower incidence of twins is explained by the natural
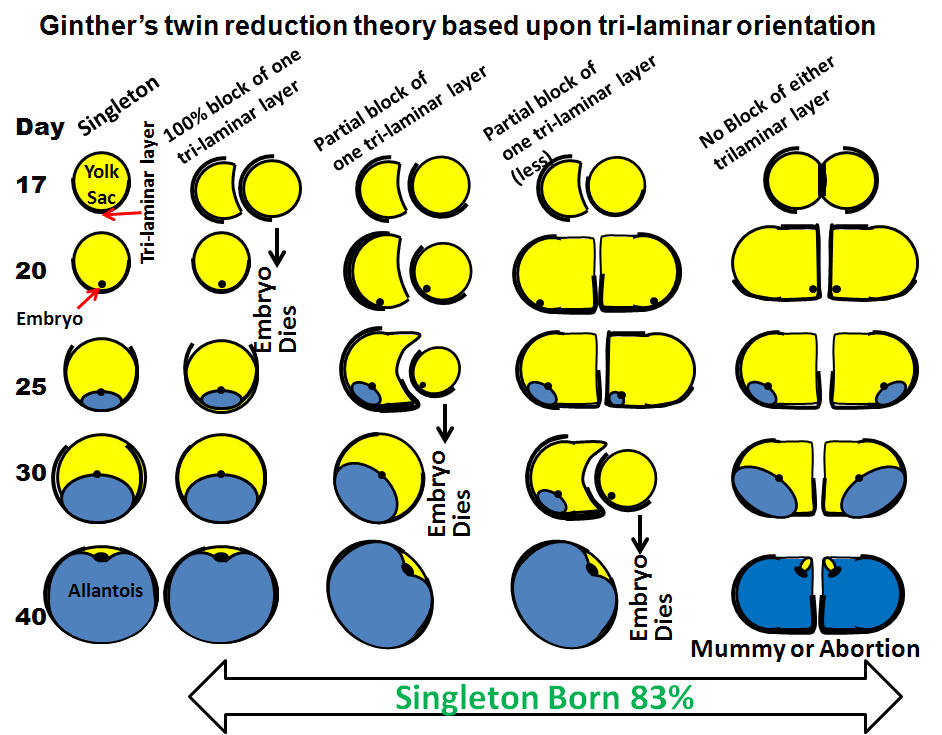
reduction hypothesis developed by Ginther.
- Natural reduction results
when both vesicles become fixed in the same horn (unilateral
fixation).
- The developing embryos have a trilaminar portion of the
trophoblast and a bilaminar portion.
- The trilaminar portion is
responsible for uptake of nutrients and gas exchange and if the
trilaminar portion of one vesicle gets covered by the bilaminar
portion of the other, it will die.
- Therefore the natural reduction
hypothesis explains why in the majority of twins, one vesicle dies
and the other survives.
- If one vesicle becomes fixed in each horn
(bilateral fixation) neither vesicle will die and both twins
survive, at least for a period of time.
- Eventually, there is
inadequate uterine capacity (placental insufficiency) and fetal
death with subsequent abortion or mummification ensues.
- Diagnosis of twins is best accomplished with
ultrasound.
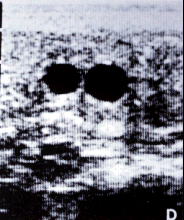
Two vesicles on ultrasound examination.
- A high degree of accuracy can be achieved. The
initial pregnancy exam should be performed at 14 d post ovulation.
If 2 CL's are observed on ultrasound, but only one vesicle is seen,
repeat the exam in a few days. Rectal palpation is not as accurate
as ultrasonography. If the embryos are in different horns, 2 bulges
are palpable from 20-50+ d but
if they are in the same horn they may be difficult to detect. Other
methods of detection such as elevated eCG levels or fetal EKG are
less reliable, more difficult and do not provide information early
enough to manage the situation properly. Unfortunately, when mares
are bred without proper supervision, a diagnosis of twins is often
made at the time of abortion.
Management
- The AVMA has established liability guidelines
regarding twins which it may be wise to review.
- Various management guidelines have been
proposed to handle the problem of twins and there are some points of
discussion.
- Some persons recommend to avoid breeding when
multiple follicles present. However, this will reduce the overall
pregnancy rate and delay conception until later in the season. Most
breeders won't be happy with this.
- Others recommend "Splitting
follicles" or breeding between ovulations. This is not a good
idea in my opinion because you're breeding after ovulation when
progesterone is beginning to rise, uterine defenses are less
effective and mechanical clearance is becoming less effective.
- In my opinion, it is best to breed regardless
of whether or not 2 follicles are present and worry about twins when
and if they occur.
- The important point is to perform the pregnancy
exam early, before fixation occurs, when one vesicle can be easily
crushed and the likelihood of the other surviving is high.
- At any rate, it is important to diagnose twins
before the endometrial cups are established. Once the endometrial
cups are in place (about 30 days), if pregnancy loss occurs (for example as a result
of a twin reduction attempt) the mare will not cycle normally and
the chance of getting her back in foal that breeding season is
slight.
- Others recommend waiting for natural reduction.
Although Ginther estimates a 5 in 6 chance of reduction to a
singleton pregnancy by 45 d, this is based on assumptions of a 20%
double ovulation rate and a 70% conception. If either rate is higher
, the incidence of twin pregnancy could be higher. Moreover, if a
mare owner has only one or two mares or it happens to be his best
mare that has twins, odds may not be as important as reality.
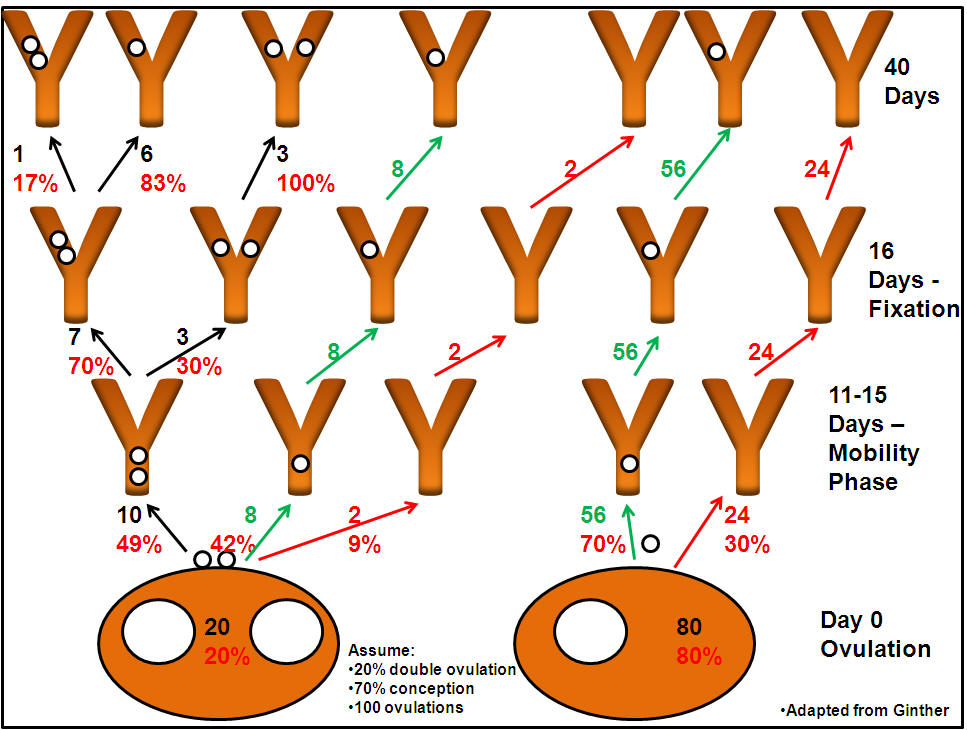
Ginther's 'Twinning Tree'
- Manual rupture - before 16 days
- Manual rupture of one vesicle as early as
possible (i.e. before 16 d) is the best management practice in my
opinion. When twins aren't discovered until too late, other options
such as ultrasound guided twin reduction or therapeutic abortion can
be discussed with the mare owner.
- Aspiration of one conceptus
- This involves a transvaginal aspiration of the fluids from one conceptus.
- A heavy sedative is given
- A transvaginal probe is inserted into the vagina and a the conceptus visualized
- A long needle is inserted through the vagina, into the fetus and all the fluid is quickly removed using an aspiration pump.
- The success rate is not good (30%). A theory on the problem is that the fluid leaks from the aspirated fetus and undermines the other fetus, thereby killing it too.
-
Twin Reduction by Cervical Dislocation (as told by Karen Wolfsdorf)
-
Best time to perform surgery is between 75 to 85 days
-
Ultrasound to locate and identify fetuses, choose the one with the least surface area or closest to the tip of the horn
-
Give Banamine pre-op
-
Give 0.5 cc detomidine
-
Clip and give local block
-
Give another 0.5 cc detomidine
-
Give 1.0 cc propantheline (This is critical – if skip this, uterus will be too turgid to be able to perform task - availabel from HGD Pharmacy)
-
Grid incision (big enough to permit arm in abdomen)
-
Locate fetus, dislocate cervix by rolling between thumb and second finger (bigger fetuses – sort of like popping a cork on a bottle)
-
Routine closure
-
Post-Op Banamine q 12 h for 2 d
-
Double dose Regumate for 30 d
-
Pen/Gent for 3 d, then 7 more d of SMZ
Fescue toxicity
- Much of the tall fescue is infested with an
endophyte (Neotyphodiun,
formerly Acremonium, coenophialum).
- Fescue infected with this endophyte actually
performs better than noninfected fescue.
- The problem occurs when
mares graze this fescue during gestation.
- Abortion, stillbirth, prolonged gestation,
dystocia, thickened placenta, agalactia, increased mare mortality
and weak foals result.
- Abnormal cyclicity, lower d 14 pregnancy rates,
and increased EED are also seen in mares grazing on infected fescue.
- The endophyte acts like a dopamine agonist.
- Treatment is aimed at either remove the mares
from the fescue at 300 d of gestation which will prevent problems or
treating affected mares with a dopamine antagonist such as
domperidone.
Trauma in late pregnancy
- To cause pregnancy loss, the trauma would have
to be quite severe and there are few documented cases. It is
unlikely that a bump would cause any problem.
Torsion of umbilicus
- The equine umbilical cord is quite long and
tortuous. It is normally twisted in utero. To be significant and be
implicated as the cause of an abortion, the torsion must be
accompanied by edema and congestion indicating impaired blood flow.
Inadequate placental attachment/placental
insufficiency
- Nutritional support for the first 35-40 d is
from the yolk sac and histotrophe.
- Initial attachment of the
placenta begins around d 45 or 60 but is not complete until 100 (+)
days and is delayed in mares with significant endometrial pathology.
- Twins, body pregnancy, and mares with
endometrial atrophy or fibrosis are examples of situations where
inadequate placental attachment occurs.
- In mares which have a long
gestation (e.g. >365 d), and deliver a small foal, especially
older mares or those with history of endometritis, this may indicate
retarded fetal growth resulting in nutritional deprivation of the
fetus.
- Slower maturation of the fetus and later maturation of the
systems involved in initiation of parturition are responsible for
the prolonged gestation.
- This is one of the reasons it is important
to examine the placenta after delivery. Areas of hypoplastic or
aplastic villi may be observed which may lead to further diagnostic
measures (biopsy of corresponding areas or hysteroscopy) or aid in
management decisions.
Nutrition
- Poor nutrition has been associated with
pregnancy loss between 7-13 wks gestation and has been advocated by
some as a tool for managing twin pregnancy. This area should be
re-examined now that ultrasound is available.
- Good nutrition ("Flushing") caused by
a change in pasture or sudden lush growth of pastures has been
associated with relaxation of the cervix, possibly allowing an
ascending infection. Estrous behavior and breeding if a stallion is
present has been observed under these circumstances. Natural
breeding may result in an ascending placentitis as the stallion
normally ejaculates into the uterus.
Transport:
- although horse owners worry about it, has never
been associated with causing abortion.
Mare Reproductive Loss Syndrom
- Kentucky in 2001
- Early loss - 90 days
- Associtated with
- More time in pasture
- Eastern Tent Caterpillars
- White Clover
- Cherry Trees
- Bred during February
- Larger farms
- Pasture feeding hay (increased
exposure to other causes?)
Prevention of
abortion
- Vaccination of mares against herpesvirus at 5,
7 and 9 months of gestation and against EAV when indicated will
reduce the chance of abortion.
- A Caslick's suture should be placed
if indicated by perineal conformation.
- Pregnant mares should be separated from other
horses, especially from new stock, young stock, etc.
- Prophylactic progestogen (altrenogest) may be
administered if indicated, i.e. in situations where endogenous
prostaglandin release is likely.
- Although there are few instances
where the need for exogenous progestogen can be documented, and
overuse cannot be condoned, altrenogest is safe to use in pregnant
mares and
there may be circumstances where it can be viewed as cheap
insurance.
|


















 Equine
Index
Equine
Index