Semen
Evaluation
  
Semen must be evaluated as soon as possible
after collection, because changes in temperature, exposure to light, and
exposure to any type of chemicals, lubricants etc. can change sperm
motility and adversely affect fertility. Motility
Motility should be examined as
soon as possible, as motility is the most influenced parameter in the
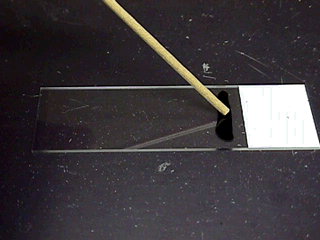
semen analysis. Use a wooden stick to handle semen, because a wooden
stick is thermo-neutral and will not cold-shock the sperm cells.
-
Gross motility is examined first.
-
Mix the semen
sample with a wooden stick, as motile sperm cells will try to swim
upward and dead cells will settle to the bottom.
-
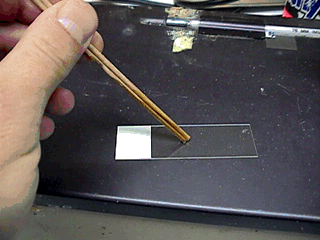
For gross motility use
2 wooden sticks to place a drop of semen on a warm slide.
-
Do not use a cover slip and examine the cells under a 10X objective.
-
The motility is
judged by the swirling motion of the sample.
-
The swirling pattern will
definitely indicate that there are cells alive.
-
The swirling looks like
currents and eddies (like a fast motion weather map).
-
Assessment of
individual cells cannot be made as live cells will carry the dead cells.
-
The gross motility will tell you though that the cells are alive, and if
the cells are dead when you examine them for individual motility there
has been a handling problem.
- Gross - swirl
pattern in bull, ram and buck.

Gross Motility
- Individual
motility is examined next.
- Individual motility checks for the
progressive movement of the sperm cells.
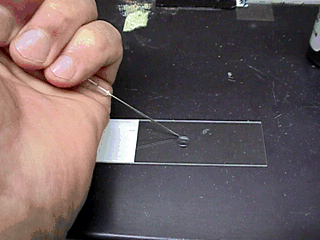
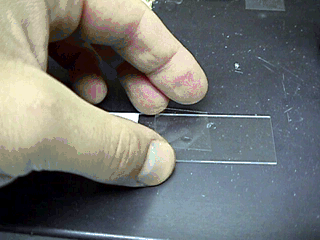
- Make the sample by placing a
drop of diluent (saline or Na citrate) on a warm slide.
- Place a small
amount of semen into the saline.
-
You want about 10 cells/high power
field in order to accurately estimate the number of cells that are
progressively moving across the field.
-
Then place a warm cover slip on
the drop.
-
Examine the sample under high dry (40X) power.
-
You must
examine the sample quickly as the motility changes very rapidly with
heat, light, and cold.

-
The motility is a very subjective measurement and is
affected by many things, such as diluent (it may be old and hypertonic
etc.), cold, the glassware, urine, soap, prostatic fluid, seminal pH,
and ion composition.
-
Computerized sperm motility computers (CASA) are
available for $30-50000
- Objective, but they probably do not tell us
that much more than by eye.

Individual Motility
Sperm morphology
-
Morphology is usually examined with an eosin-nigrosin
(Society for Theriogenology) stain (background stain) to highlight the
cells.
-
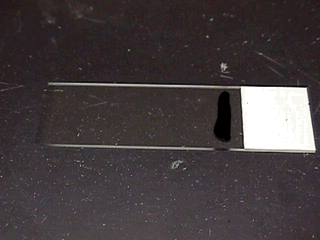
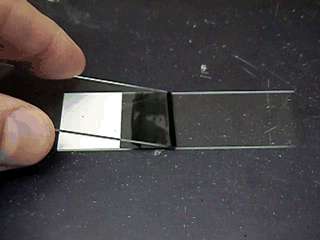
The slide is made by painting a drop or line of stain on a warm
slide, then using a wooden stick to place a small amount of semen
into the stain; the semen and stain are mixed using another slide, then
slowly push the second slide through the stain and across the first
slide while pressing firmly down. You should be making a 'bad' blood
smear.
-
The goal is to get a dark background, as the stain is a background stain and is not intended to stain the cells.
-
In fact, some cells
will stain red, but this makes no difference in our evaluation.
-
You want
the cells to be close, but not overlapping.
The final slide should have
dark and light areas that allow you to view different colored backgrounds as needed when examining the slide.
-
Examine the cells under 1000X (oil)
to fully assess the morphology.

-
Count 100 cells and, in a practice situation, you can
just differentiate normal from abnormal cells.
-
Using eosin-nigrosin all
the cells will appear flat, as if looking at your hand. In order to do a
full spermiogram, differentiate the abnormalities by type.
-
A phase
contrast microscope can be used, but is rare in a practice situation.
-
A
phase contrast mount is made by placing a small amount of semen in
formal-buffered saline to kill and preserve the cells.
-
Then place a drop
of the formal-buffered saline sample onto a slide and place a cover slip
over the drop.
-
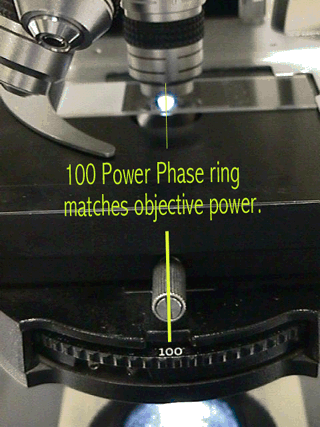
You need a phase contrast microscope to
examine the specimen. In using the phase-contrast microscope, match the
phase ring with the power that you are using.
-
The phase contrast acts
like a 'stain', however the cells will float by instead of lying flat.
-
The cells now look like your hand from the top and side views.
Abnormalities
-
Abnormalities
are classified as primary and secondary.
-
Primary abnormalities are
thought to arise in the testes, whereas secondary abnormalities arise in
the epididymis or ejaculate.
-
Secondary abnormalities may be just as
serious primary.
-
Primaries abnormalities decrease through transit and
secondary abnormalities increase through transit.
-
Major
and minor may be a better classification.
-
Major problems cause Early Embryonic Death or prevent fertilization.
-
For
example, an acrosome problem prevents zona entry, and a nuclear problem
leads to nonfertilization or EED.
-
Minor
problems such as tail abnormalities etc. stop sperm movement, so the
sperm cell cannot get to egg.
-
Some abnormalities are compensable and
some are non-compensable.
-
Problems with sperm cell
examination.
-
The light microscope cannot always detect abnormalities.
-
Abnormal
sperm cells indicate cellular damage, but the relationship to fertility
is only circumstantial.
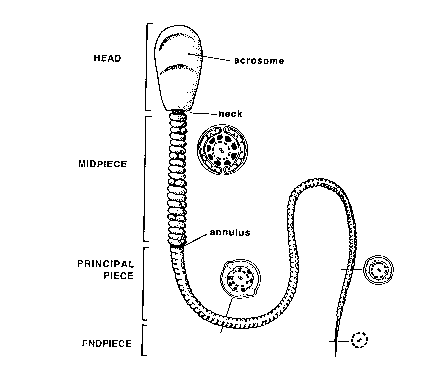
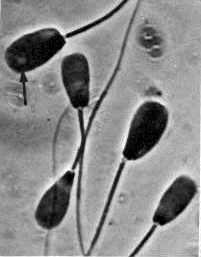
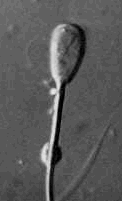
Normal Sperm Cell above
Chart of Sperm Cell
Abnormalities
below
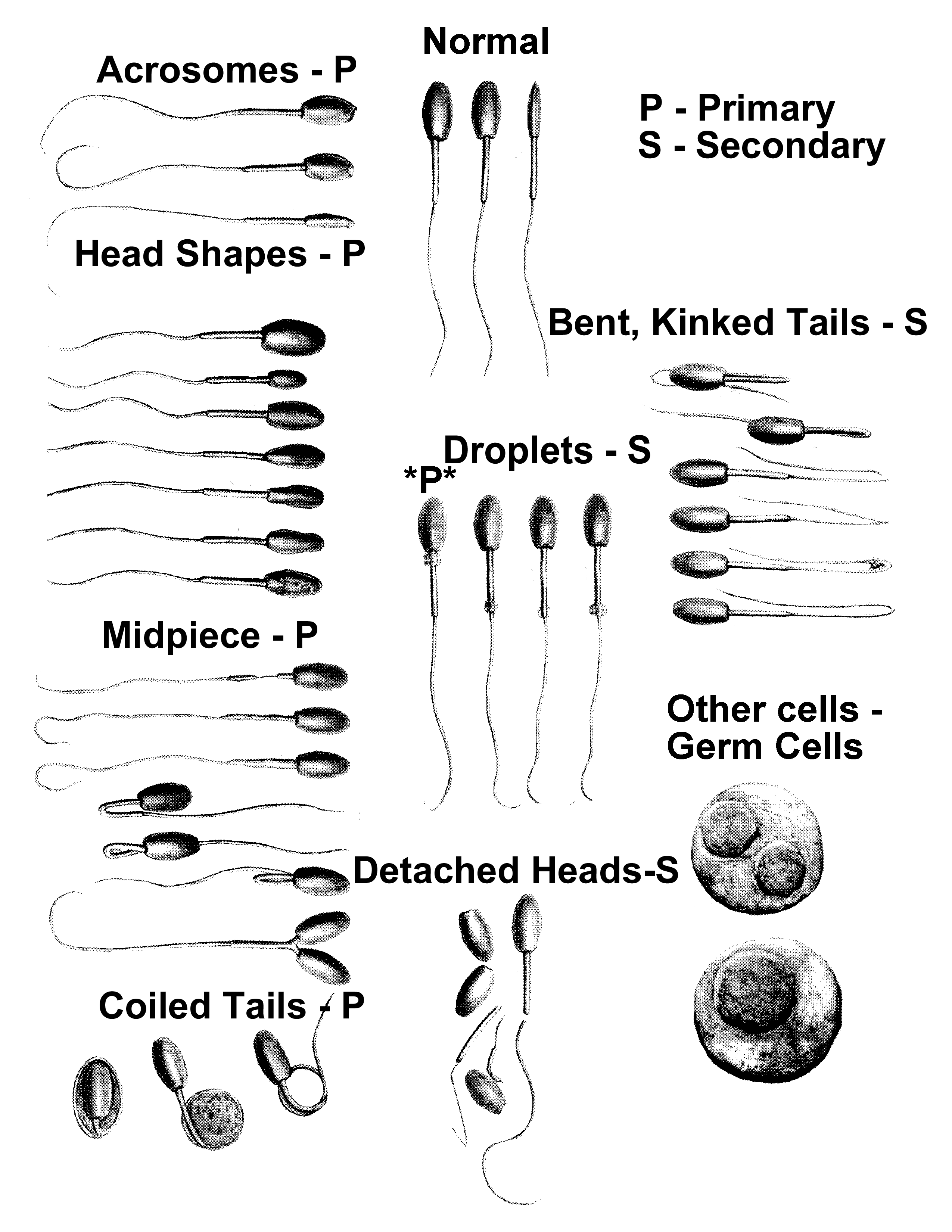
Click to enlarge
Abnormalities of the sperm cells include:
-
Decapitated sperm (not pictured)- the basal plate is defective;
tails move (wrap around drops); 100% cells involved; occurs in
epididymis (secondary)
-
Loose heads (not pictured)- these may be increased on the first
ejaculate (rusty load) because the tail attachment is frail.
-
Knobbed or flat
acrosome (not pictured)- the acrosome folds over itself of the apex of the acrosome is
knobbed or flattened. When 20% of the cells have acrosome problems the
result may be infertility in the bull. This condition may be hereditary
in Charolais, Hereford, and Holsteins.
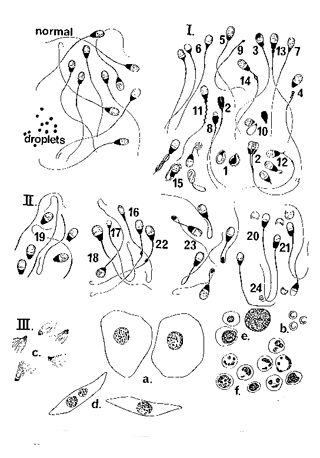
 Wrinkled acrosome - this may reflect a nuclear
problem which prevents zona attachment by the sperm cell. It is a rare
condition. Wrinkled acrosome - this may reflect a nuclear
problem which prevents zona attachment by the sperm cell. It is a rare
condition.
 Pyriform and tapered heads - the
nuclear material is poorly distributed. The defect may be subtle. Pyriform and tapered heads - the
nuclear material is poorly distributed. The defect may be subtle.
 Giant or small heads - This a nuclear problem. If
the head is twice normal size the cell is a giant cell. Giant or small heads - This a nuclear problem. If
the head is twice normal size the cell is a giant cell.
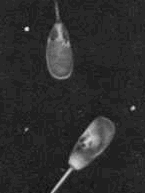
 Nuclear vacuoles - These distort the shape of the
head. Nuclear vacuoles - These distort the shape of the
head.
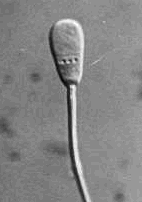
 Diadem defect - With this you see invaginations in
the nucleus, mostly by the post nuclear cap. The pit lacks DNA. The
condition may be associated with stress in bulls and may come and go as
stress changes. Diadem defect - With this you see invaginations in
the nucleus, mostly by the post nuclear cap. The pit lacks DNA. The
condition may be associated with stress in bulls and may come and go as
stress changes.
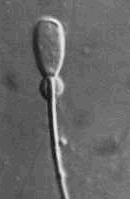
 Dense proximal droplets - This arises in the
epididymis and indicates maturation problem. Dense proximal droplets - This arises in the
epididymis and indicates maturation problem.- Stump tail defect (not pictured) - this is an axonemal problem. It
looks like a cytoplasmic drop and has a poor prognosis. The incidence
increases with age. Midpiece defects - You see
lumps on the midpiece that can be confused with proximal drops. Coiled
mainpiece - The mainpiece is coiled within the plasma membrane.
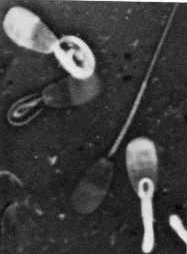
 Dag defect - This is a sterilizing defect that occurs
in the epididymis so is it is actually a secondary abnormality, but it
is a major defect. The condition is inherited and the axoneme is
disrupted (fibrils and helix). You see split, shattered, or fractured
midpiece. The tail may coil and the motility is low. Dag defect - This is a sterilizing defect that occurs
in the epididymis so is it is actually a secondary abnormality, but it
is a major defect. The condition is inherited and the axoneme is
disrupted (fibrils and helix). You see split, shattered, or fractured
midpiece. The tail may coil and the motility is low.- Coiled midpiece (not pictured). This is an epididymal defect, but is
a major defect. The midpiece problems (not Dag).
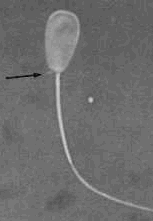
 Abaxial midpiece - the implantation fossa is
defective. You may see abaxial or double midbpieces; There is generally
a low incidence and fertility is not affected; (normal in stallion). Abaxial midpiece - the implantation fossa is
defective. You may see abaxial or double midbpieces; There is generally
a low incidence and fertility is not affected; (normal in stallion).
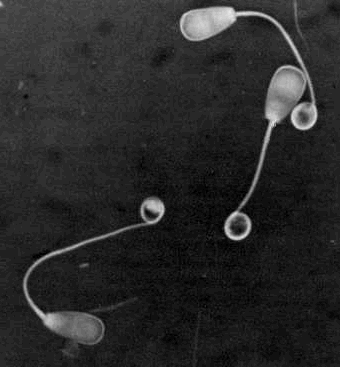
 Coiled
mainpiece - The mainpiece is coiled within the plasma membrane. Coiled
mainpiece - The mainpiece is coiled within the plasma membrane.
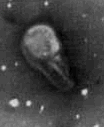 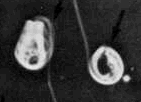 
 Teratospermia - the entire cell is degenerative. Teratospermia - the entire cell is degenerative.
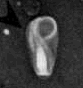
 Bent
tails - the bend in the tail may include a droplet which may be in the
membrane. Bent
tails - the bend in the tail may include a droplet which may be in the
membrane.
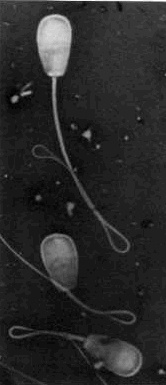
 Physiologic
(distal )droplet - some consider this a minor
defect, but in fact it may be a major defect. These cells do not freeze
well because the water in droplet crystalizes and ruptures the cell
membrane. Physiologic
(distal )droplet - some consider this a minor
defect, but in fact it may be a major defect. These cells do not freeze
well because the water in droplet crystalizes and ruptures the cell
membrane.
Temporal progression of sperm abnormalities after 4
days of scrotal insulation.
| Days after scrotal insulation |
Sperm abnormalities in the ejaculate |
Days return to normal |
| 7-11 |
Distal midpiece reflex, Proximal Droplets |
40 |
| 11-15 |
Mitochondrial Sheaths, Detached heads |
35 |
| 18 |
Knobbed acrosomes |
25 |
| 20 |
Nuclear Vacuoles |
22 |
| 22 |
Pyriform heads |
20 |
| 23 |
coiled principal piece |
19 |
| 42 |
normal |
0 |
Sperm count
- In
bulls and rams, the sperm count is estimated by measuring the scrotal
circumference. The scrotal circumference measurement is used because
electroejaculation does not give a physiologic sample that can be
reliably counted.
- Hemacytometer
method
- To count the concentration of semen sample a
hemacytometer can be used.
- The hemacytometer is loaded with a 1:100
dilution of semen (see below how to make a 1:100 dilution,
- a hemacytometer coverslip is placed over the
chambers
- the chambers are filled
- the sample
allowed to settle,
- all the sperm heads in the middle big square (the
square with 25 smaller squares (....and another 16 squares within each
of these 25) within the triple lines) are counted.
- The number of sperm
heads counted in a single chamber is multiplied by 106 to give the
concentration of cells/cc.
- Both chambers of the hemacytometer should be
counted and the numbers should not differ by more that 10%.
- The coverslips are expensive so do not
break them or dispose of them!
- Sound
confusing...look at the diagram.

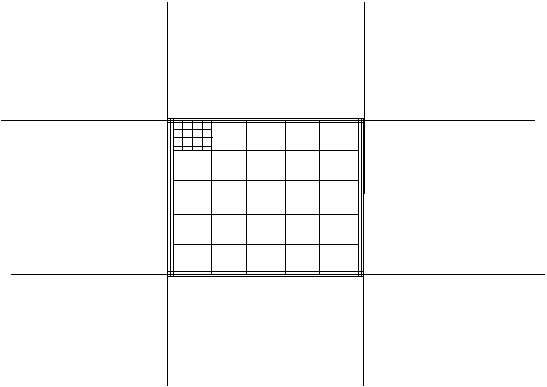
- The
hemacytometer grid has 9 large squares. The central square has triple lines
around it. Inside the triple lines are 25 smaller squares (also bounded by
triple lines). Within each of the 25 squares are 16 squares (only one of the
25 cells is illustrated having the 16 smaller squares. This is illustrated
below, however the triple lines are only shown on the outside edges of the
middle big square.
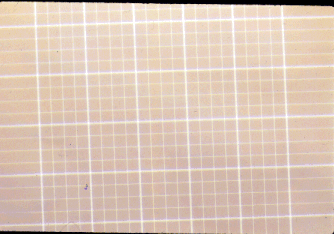
Here
is an actual picture of the grid of the 25 squares in the middle big square.
They are all bounded by triple lines. Count every sperm head bounded by
triple lines.
 Here
is a picture of one of the 25 squares with the 16 squares inside. 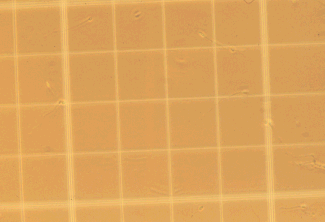
For example, if each of the dots represented a
sperm head, the count on the grid below would be 13 x 106
cells/ml. 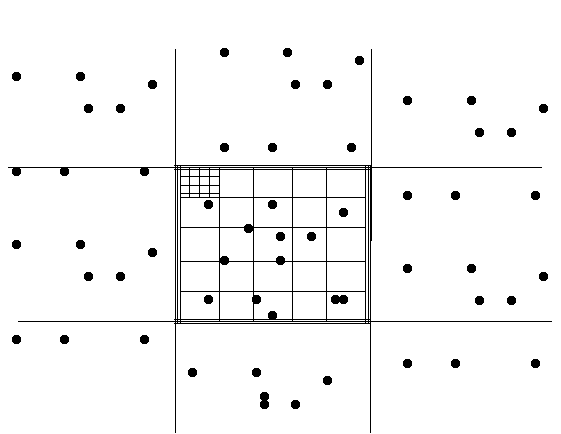
-
The 1:100 dilution can be made using a Unopette
diluter or a hand dilution.
-
The Unopette is easier, but sometimes the
cells will clump, so an accurate count cannot be made.
-
If using the
Unopette, first puncture the bottle with the sharp point, then draw up
the semen via capillary action into the capillary tube.
-
The bottle is
then squeezed and the capillary tube inserted into the bottle and the
semen sucked into the bottle.
-
You can turn the capillary tube over and
use it as an applicator to load the hemacytometer chambers.
- We no longer use the Unopette at LSU

-
A hand dilution is made by:
-
diluting 1 part semen with 9 parts
formal-buffered saline to make a 1:10 dilution,
-
then taking 1 part of
the 1:10 dilution and adding 9 parts of formal-buffered saline to make a
1:100 dilution.
-
This is a little more time consuming, but the cells tend
not to clump, so you get a more accurate sperm count.
Click on the above diagram to see a
slide show demonstration.
-
A spectrophotometer can be used to count stallion
semen (and possibly dog semen if the correct software is used).
-
Basically, the machine measures the amount of light that passed through
a sample and calculates the concentration of cells that the density
reflects.
-
Using our machine:

- SpermAcue
- Spectrophotometric method
- Canine and equine

Total sperm numbers.
- NucleoCounter
- Cell membrane disrupted
- Dye stains DNA
- Digital image created
- Cells counted
- Can also be used to count intact membranes by
not disrupting the cells first

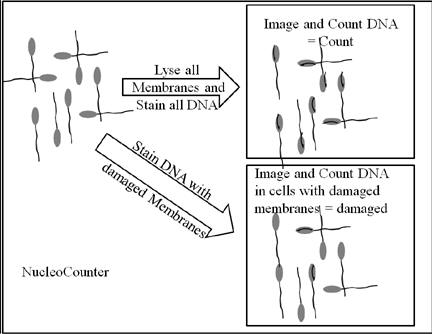
- Multiply
the concentration X the volume to give the total number of cells in the
ejaculate.
- Take that number and multiply by the percent progressively
motile cells to get the total number of progressively motile cells.
- Take
that number and multiply by the percent normal cells to get the total
number of normal, motile cells.
- For example: 100
cc X 50million cells/cc = 5 billion cells 5
billion cells X 50% motility = 2.5 billion motile cells 2.5
billion motile cells X 50% normal = 1.25 billion normal, motile cells.
- Total sperm numbers are used in stallions and dogs, but not in bulls,
rams or bucks.
PREPARATION AND SHIPMENT OF FRESH, COOLED, STALLION SEMEN
- Advantages of transported fresh semen
- All the advantages of on-farm artificial insemination with fresh
semen
- Reduced chance of disease transmission
- Increased book size for stud owner
- Less chance of injury to horses and handlers
- Less expensive to ship semen than horses
- Decreased cost of broodmare care at studfarm?
- Decreased stress and chance of injury to mare and foal due to
shipping
- Breeding can continue while stallion is engaged in other
activities
- Increased availability of superior stallions or uncommon breeds
- Semen evaluation possible at time of breeding
- Disadvantages of transported fresh semen
- Lower fertility with some stallions
- Lower fertility with prolonged shipping times
- May be increased cost realized due to processing semen
- May be increased cost due to lower per cycle pregnancy
- Requires additional equipment and training for semen processing
- Requires better mare management
- Requires good stallion management
- Requires good communication between all parties
- Requires advance planning, semen may not be available every day
- Factors influencing success with transported semen
- Pregnancy rates with transported, cooled semen are similar to
those obtained after using fresh semen, provided mare management,
semen quality and semen handling are good and shipping times are
relatively short (< 24 hrs). Shipping times greater than
24 hrs are associated with some degree of reduction in pregnancy
rates, possibly as much as 50%. For these reasons, proper techniques
of semen evaluation, extending and packaging are essential.
- Semen quality / stallion fertility
- Motility, concentration, volume and morphology before extending
- Quality after storage / shipment
- Type of extender used
- Concentration, dilution ratio of extended semen
- Type of packaging system used
- Cooling rate
- Lowest temperature reached
- Ability to maintain stable temperature
- Duration of transport
- Number of normal, motile sperm inseminated
- Timing of insemination: ability to predict ovulation, use of hCG
or GnRH analogues to induce ovulation and avoid need for repeat
shipments
- Mare management / fertility
- USE OF SEMEN EXTENDERS AND SHIPMENT OF FRESH,
COOLED SEMEN
- The shipment and use of fresh cooled
semen is experiencing increasing popularity. Restrictions enforced by
the various breed associations are the main factor preventing its
widespread use. Semen extenders are an essential component of fresh
cooled semen.
- Semen extenders are an important adjunct to an artificial breeding
program. Use of semen extenders makes it possible to ship semen
overnight while preserving fertility. Their beneficial effect is
also used at times in natural breeding by infusing the extender into
the uterus in conjunction with mating.
- When an extender is added to fresh semen, it is not unusual to see
an initial increase in motility. As time passes, motility of the
extended semen remains higher and is maintained longer, than the
motility of the unextended, or raw, sample. In addition, semen
extenders containing antibiotics help to reduce the contamination
introduced into the mare's uterus at breeding. For these reasons,
semen extenders may improve the fertility.
- Semen extenders provide substances for the metabolic activity of
the spermatozoa, buffer against changes in acidity and protect
against cold shock. Glucose is the primary nutritive component of
most extenders. Depending on the particular recipe, egg yolk or milk
normally provides the protective effect against cold shock. If milk
is used, either half & half, which is heated in a double boiler
and the scum removed, or nonfat dry skim milk are usually used.
Nonfat skim milk is very easy to use, however, only brands without
added preservatives are suitable. Most commercially available
extenders rely on non-fat dry skim milk as a base. Antibiotics are
usually added to the extender to inhibit growth of bacteria in the
semen during storage. Studies indicate that the antibiotic polymyxin
B is not suitable for storage of semen, therefore it is not used in
extenders for transported semen. Although other studies have
indicated slight differences in motility after storage with various
antibiotics, from a practical standpoint antibiotics such as
ticarcillin, gentamicin or amikacin all give satisfactory results
and are commonly used.
- Osmolarity and acidity are critical factors in the preparation of
an extender. After preparing an extender, both pH and osmolarity
should be checked before use. If instruments are not available to
check pH and osmolarity, the extender should be tested to verify
that sperm viability is maintained before the extender is used for
shipping. Some antibiotics may significantly alter the pH of the
extender and sodium bicarbonate must be added to restore it to a
suitable range.
- Semen extender may be prepared in large quantities and then frozen
and stored in smaller aliquots, such as 100 ml, for later use.
Properly stored, the semen extender will be preserved for 3 to 6
months and reduce the need for frequent extender preparation.
Commercially available extenders are very easy to use and are
formulated to provide the correct pH and osmolarity. They usually
consist of a packet of dry ingredients and a small vial of diluent
which are mixed together at the time of use. Most are available in
convenient 100 to 125 ml sizes so that extender is made as needed
rather than pre-made and frozen.
- Sperm will quickly metabolize available substrates in seminal
plasma so motility will decrease rapidly. Therefore, after the semen
is collected, it should be mixed with extender as soon as possible,
preferably within 10 or 15 min. Ideally, the ratio of semen to
extender should be at least 1:2, and a ratio of 1:4 or 1:5 is
preferable. Nevertheless, it is the final concentration of sperm in
the extended sample that is the critical factor. Longevity of the
sperm cells is maximized if extender is added to give a final
concentration of sperm cells of 25 - 50 million/ml. With an adequate
extender at a proper dilution, sperm will retain their fertilizing
capability for up to 24 hours at room temperature (20oC),
depending on the individual stallion. Therefore a semen:extender
dilution factor should be calculated based on the initial
concentration of sperm to give a final concentration of 25 - 50
million sperm/ml before shipping.
- For example:
-
Concentration at collection = 267 million/ml
-
Desired concentration = 50 million/ml;
-
267 / 50 = 5.3 total parts semen + extender
-
5.3 total parts - 1 part semen = 4.3 parts
extender needed
- Therefore, use 5 parts extender : 1 part semen to give a final
extended concentration of 45 million sperm / ml
- If a stallion provides an ejaculate with a low concentration, so
that dilution at a 1:4 ratio, for example, would result in the final
concentration being less than 25 million/ml, centrifugation is
recommended. Centrifugation can be used to concentrate the semen so
that dilution at a recommended ratio can be achieved while
maintaining a concentration of 25 - 50 million/ml.
- Recommendations for centrifugation are to begin with a force of
500 X g for 10 minutes. Less time or fewer g’s will result in less
damage to the sperm from the force of centrifugation but a softer
pellet and more viable motile sperm in the supernatant that will be
discarded. Centrifugation for a longer time or at higher g’s will
achieve a better recovery and minimize sperm losses in the
supernatant but result in more damage to the sperm cells.
Centrifugation technique can be altered within a range of g’s and
times to achieve good recovery while minimizing cellular damage.
- After centrifugation, the supernatant is removed and discarded.
Studies have shown that a small portion (a minimum of 5%) of the
seminal plasma must be left with the sperm to preserve viability.
The pellet is then resuspended using sufficient extender to achieve
a final concentration of 25 - 50 million/ml.
- Preservation of semen quality depends to a large extent on the
initial quality of the semen, and varies from stallion to stallion.
- Determination of insemination dose
- Prior to shipment of semen from a stallion (and periodically
during the breeding season) it is advised to do a trial run with the
chosen shipping system and determine the motility after 24 and 48
hours of storage. This allows you to determine the "recovery
rate" and make adjustments in the number of sperm shipped so
that an adequate insemination dose will be provided when the semen
reaches its destination.
- In addition, it is advisable to maintain a record of the
collection data. Such information may help in determining the
collection or shipping frequency, shipping dose and number of doses
to expect from an average collection.
- The recommended insemination dose is usually 500 million
progressively motile (normal) sperm, although acceptable pregnancy
rates can be achieved with as few as 250 million normal,
progressively motile sperm. The volume of the inseminate is not
critical. Although pregnancy can be achieved with very small
volumes, the recommended minimum volume used for insemination is
usually 10 ml. Usual insemination doses with fresh cooled semen
range from 20 to 120 ml. Although some veterinarians are reluctant
to inseminate volumes greater than 60 ml, studies have shown no
decrease in fertility when larger volumes were inseminated, provided
the inseminate was not real dilute.
- To determine the volume of semen to package for shipment, divide
the desired number of progressively motile sperm (usually 500
million) in an insemination dose by the product of the concentration
times percent motility at the end of the storage period. For
example, if we have extended our semen to a concentration of 50
million/ml and our motility after transport is 40%, we should
package at least :
- 500 / (50 X .40) = 500 / 20 = 25 ml. Unless a stallion is in very
high demand it is best to package more than the minimum amount
needed. For example, with the above stallion, packaging 50 ml would
insure that more than adequate numbers of sperm were available for
fertilization of the oocyte. Furthermore, a more conservative method
is to include percent normal morphology into the equation. For the
stallion above, if normal morphology is 70%, the equation becomes:
- 500 / (50 X .40 X .70) = 500 / 14 = 35 ml
- Semen Packaging
- The pre-warmed extender should be added slowly to the semen. The
extended semen should be placed in a container such as a Whirl-Pak
bag, from which air can be excluded, and sealed. This container
should then be placed in a second container from which air is
again excluded and the second container also sealed.
- It has become commonplace for some people to place two
insemination doses in the shipping container, intending one to be
used upon arrival and the other to be used the following day. In
fact, there is no physiological basis for such practices. The
ideal place to store spermatozoa is in the mare’s oviducts.
Man-made semen transport devices are a means to transport semen
from the stallion to the mare without transporting horses. They
are not meant to take the place of the mare as a site of sperm
storage until fertilization. All semen received in a transport
device should be inseminated into the mare at the time of arrival.
This should be kept in mind when preparing the semen for shipment.
- Ideally, the semen should be extended and placed in the cooling
device within 10 minutes of collection. The rate at which extended
semen is cooled is critical. If the cooling rate is too fast or
too slow, sperm viability is decreased. A cooling rate of -0.05 to
-0.1 C/min is desired between 20 and 5 C. Ideal storage
temperature is 4-6 C.
- Numerous types of containers for fresh, cooled semen are
commercially available. Reusable containers such as the Equitainer
as well as inexpensive disposable systems are in use worldwide.
The systems vary in their cooling rates, minimum temperature
reached, time required to reach the minimum temperature, length of
time they are able to maintain the minimum temperature, etc.
Performance of the different systems also varies depending on
environmental conditions. Some of the disposable systems such as
the Equine Express and BioFlite compare very favorably with the
Equitainer system, while others such as the ExpectaFoal compare
less favorably, reaching temperatures below 0 C.
- Some reports indicate sperm viability is decreased due to
contact with the rubber plunger on syringes. Syringes constructed
of all plastic are therefore recommended by some researchers. In
actual practice, however, the extended semen is not in contact
with the plunger very long and the numbers of sperm cells being
inseminated are great enough that toxicity from the syringe is
probably of little clinical significance. If semen is held in
syringes for any length of time, however, as in some types of
shipping devices, syringes of all plastic should be used.
- An information form should be included with each shipment.
Minimum information required on the form includes stallion
identification, date of collection, concentration and volume of
inseminate shipped (i.e. numbers of sperm), initial motility, type
of extender used, numbers of doses shipped and any special
instructions.
- Prior to shipment of semen from a stallion (and periodically
during the breeding season) it is advised to do a trial run with
either shipping system and determine the motility after 24 and 48
hours of storage. This allows you to make adjustments in the
number of sperm shipped so that 500 million motile sperm will be
provided when the semen reaches its destination.
- Use of some of the more common packaging systems are described
in more detail:
EQUITAINER

- The Equitainer system is a very durable, hard plastic
insulated container. In the center of the Equitainer is a
well. The system is designed to cool a volume of 120 to 170
ml of fluid at the optimum rate. The extended semen sample
is nestled in ballast bags in a plastic cup so that the
total volume of the semen plus the ballast bags is between
120 to 170 ml. Each ballast bag holds 60 ml of a colored
fluid. Ballast bags should be warmed in an incubator (37oC)
for 4 hours before use. The plastic cup containing the semen
is placed into the "isothermalizer". One or two
(depending on the model of Equitainer) specially designed
freezer packs are placed in a plastic bag and loaded into
the well of the Equitainer. The isothermalizer is then
loaded into the well on top of the freezer packs. Records
should be enclosed that identify the source of the semen and
for the person breeding the mare to fill out. The particular
forms may differ depending on the breed involved. The
container is then closed and latched. It is a good idea to
seal the container so that it will be evident if any
tampering occurs.
- A sample of the extended semen that was packaged in the
Equitainer should be kept in a similar manner for evaluation
24 hours later, as a quality control. If a second Equitainer
is not available, the cooling and storage procedure can be
simulated by placing a sample in a Whirl- Pak bag which is
then placed in a polypropylene cup. The cup is then placed
in a water bath of approximately a pint of water in a
container about 6 inches in diameter, which has been
prewarmed to 37oC. Place the whole assembly into
a refrigerator set at 5oC. After a 24 hour
interval, warm the semen to 37oC and assess
motility.
- Various models of the Equitainer system are available.
Models differ somewhat in the length of time they will
maintain the semen chilled and whether a lead shield is
present. It should be remembered, though, that shorter
storage times result in improved fertility.
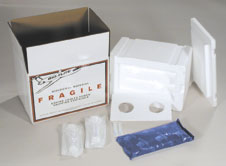
- EQUINE EXPRESS

- A number of "disposable" semen shippers are currently
available, one of which is the Equine Express. It is inexpensive and consists of a cardboard box with styrofoam inserts and compartments for the semen and a freezer
pack. The semen is packaged in all-plastic syringes. After
collection, evaluation and extension of the semen, the
extended semen is placed in an all plastic syringe. The
syringe containing the extended semen is placed in the
styrofoam box along with another syringe containing an equal
volume of water as "ballast’ to moderate the cooling
rate. Alternatively, the semen can be packaged in 2 syringes
of equal volume. A styrofoam sheet is then placed in the box
over the syringes and a specially formed freezer pack placed
on top of that. Breeding forms and other pertinent information
should be enclosed before sealing the box.
- Another inexpensive yet dependable disposable shipper is the
BioFlite. It is similar in some ways to the Equine Express, in
that it consists of a styrofoam box with a lower compartment to
hold the semen, an upper compartment that holds a freezer pack
and a styrofoam sheet between the compartments. In previous
models the semen was packed in plastic bags that were then
placed in a plastic specimen cup. In newer models of the
BioFlite the semen is packaged in all-plastic syringes.
BioFllite also makes a model for shipment of canine semen.
Made by Plastilite, Inc. Either a cardboard or plastic outer
shell is available, and either a styrofoam or special insulating
liner are available.
- Transport and Insemination
- After packaging, the container is shipped by commercial carrier
or airline to be delivered within 24 hours. Some concerns have
been raised about x-radiation of semen as it passes through
airport security. Studies examining the effects of doses of
radiation used in airport security have found no adverse effects
on spermatozoa. However, there are indications that the airports
will soon increase the level of radiation in an attempt to improve
security and the effect of the increased level of radiation is
unknown. The Equitainer system has a lead shield in the transport
container to shield the semen from the radiation and any possible
harmful effects.
- When the semen arrives at its destination, the mare is prepared
for artificial insemination. Either while the mare is being
prepared or after she is inseminated, the semen should be examined
to determine percent motility. Care must be taken to maintain the
semen at the chilled temperature in the container until ready to
place it into the mare. The best place to rewarm the semen is in
the mare's uterus. Prewarming the semen before placing it into the
mare decreases conception rate. A drop may be removed from the
sample container and placed on a warm microscope slide on a slide
warmer. A warm cover slip is placed on top and motility estimated
in the same manner as during a breeding soundness examination.
Motility will improve as the sample is allowed to warm. The
concentration may also be determined if it is unclear how many
intended insemination doses were sent.
- Shipping
- The use of fresh, cooled semen provides a number of advantages.
It is much easier to ship a container of semen to a mare than to
ship a mare and foal to a stallion. Not only is cost
decreased by shipping semen
rather than horses, but stress on the horses is greatly reduced
also. Fresh, cooled semen allows more efficient use of a stallion,
not only for shipping, but for temporary storage on the farm to
reduce the frequency of collection. For example, a stallion can be
collected, the ejaculate extended and a portion used to inseminate
mares that day. The remainder can be cooled and used to inseminate
mares 24 to 48 hours later. Shipment of semen increases the
availability of superior stallions or stallions of uncommon breeds
and allows a stallion to breed a greater number of mares in a
season.
- Some slight disadvantages are inherent in the use of shipped,
fresh cooled semen. For unknown reasons, considerable variation
exists between stallions in the ability of their sperm to remain
viable during the cooling and storage process. For all stallions,
however, fertility is generally higher if the storage period is
shorter. If care is taken in the preparation of fresh, cooled
semen and mares are managed well, pregnancy rates using fresh,
cooled semen can be as high as those with a natural breeding
program. An important consideration if semen is being shipped in
to breed a mare is the advanced planning required. This may be
complicated by the fact that semen may not be available every day
of the week due to the work schedule of the shipping company. In
addition, use of shipped semen requires good mare management. Good
record keeping and a good teasing program are integral components
of a successful breeding program. Ability to predict ovulation is
essential in order that semen arrive before the mare ovulates, and
to avoid repeated shipments of semen during a single estrus.
Research has shown that breeding too long after ovulation results in
decreased pregnancy rates and increased early embryonic death.
PREPARATION
AND SHIPMENT OF FRESH, COOLED, CANINE SEMEN
-
Semen is analyzed
-
Extension
-
Appropriate
semen extender is added to the ejaculate, usually 1:1.
-
Semen
extenders provide an energy source and buffers that enhance the
survival of chilled sperm cells.
-
Extenders
can be obtained from commercial sources that are manufactured
exclusively for extending canine semen (Synbiotics, San Diego, CA
92127; CLONE, Chester Springs, PA 19425; Camelot Farms, College
Station, TX 77842; International Canine Semen Bank, P.O. Box
651,Sandy, OR 97055),
-
Equine
semen (Lane Mfg., Denver, CO 80231; IMV Intl, Minneapolis, MN
55430) work well and are much cheaper.
-
Homemade
semen extenders can also be prepared, however proper laboratory
techniques
-
The
extender must be pre-warmed before adding it to the semen or the
spermatozoa will suffer cold-shock.
-
The
prostatic portion of the ejaculate may have detrimental effects on
the storage of canine sperm cells,
-
We
found no detrimental effects on pregnancy rate or fecundity when
whole ejaculates were extended 1:1 and inseminated after 24 or 48
hours of storage.
-
If
the entire prostatic portion of the ejaculate is collected and the
semen is extended, the volume of the resulting extended semen may
be such that it cannot be completely inseminated without some
vaginal reflux.
-
Our research at LSU has shown (EVSSAR Estoril 2007):
-
Prostatic fluid and centrifugation have no effect on the membrane integrity of the cells when semen is extended in a commercial equine extender.
-
Semen extended in a commercial equine extender and chilled should have the prostatic fluid removed before storage and it should be stored at a relatively high concentration for maximum motility.
-
If a sample does arrive that has a large volume because the prostatic portion was included in the extended semen, centrifugation to remove the prostatic fluid yields progressive motility that is not different from removing the prostatic fluid before extension.
-
Containers
-
Containers
designed to ship semen can be obtained from many of the same
sources that provide canine semen extenders. (Synbiotics, San
Diego, CA 92127; CLONE, Chester Springs, PA 19425; Camelot Farms,
College Station, TX 77842; Bio-Flite, Anaheim Hills, CA 92807;
International Canine Semen Bank, P.O. Box 651, Sandy, OR
97055).
-
The
containers usually consist of a Styrofoam box, an ice pack, and
container for the semen.
-
These
commercially available semen containers maintain semen well enough
that acceptable pregnancy rates result.
-
Commercially
available shipping containers offer a predictable, attractive and
easy way to ship semen.
-
Many
of these containers are relatively expensive when compared to the
disposable equine shipping containers.
-
A
commercially available (although no longer marketed)
disposable equine shipping container allowed storage of
extended canine semen for up to 48 hours and the resulting
pregnancy rates were equivalent to AI with fresh semen.
-
We
have used other brands of
disposable equine semen
shippers and have had good success in maintaining semen
viability.
-
The
advantage of the equine shippers is their lower cost than the
canine semen shippers

|
 Male
Index
Male
Index Male
Index
Male
Index