Infertility
in the mare
 172-176, 179-185 172-176, 179-185
Infertility can be
divided into categories
- 1) mares that fail to cycle
- 2) mares that cycle normally but don't conceive
and
- 3) mares that cycle normally and conceive but
then suffer early embryonic death (EED)
The non-cycling
mare
- First, always rule out the possibility of
pregnancy!
- Other causes of failure to cycle include winter anestrus,
transition, prolonged diestrus, behavioral anestrus, chromosomal
anomalies, and ovarian tumors.
- The topics of winter anestrus,
transition and behavioral anestrus have been covered previously.
- A prolonged luteal phase, also called prolonged
diestrus, persistent CL or pseudopregnancy can be caused by a
variety of factors.
- Possible causes include failure of PGF release,
insensitivity to the PGF released (e.g. diestrous ovulation),
embryonic death after maternal recognition of pregnancy, etc.
- If
untreated, it can last for 60-90 d.
- This was more of a problem in
the pre-ultrasound days, but now with ultrasound and the ability to
diagnose pregnancy at 14 days, prolonged luteal phase in the
nonpregnant mare can be easily diagnosed and treated.
- Treatment is
Prostagandin.
Chromosomal anomalies
- Abnormalities of karyotype are being found more
commonly as our ability to look for them increases.
- Gonadal dysgenesis mares appear phenotypically
female. Their external genitalia are normal. They are generally
small in size (often short with a big head). Regarding sexual
behavior, in response to teasing they are passive or occasionally
exhibit irregular estrous behavior. On palpation per rectum, their
ovaries are very small, smooth and firm. The uterus is small and
flaccid (a thin band). The cervix is small and flaccid, but may
appear normal on speculum exam. Karyotyping is needed for definitive
diagnosis. These mares are sterile.
Molecular Cytogenetics Laboratory
Room 318 B
Bldg 1197
Department of Veterinary Integrative Biosciences
Texas A&M University
College Station, TX 77843-4458
Telephone: 979-458-0520
Fax: 979-845-9972
Please call in advance. Laboratory led by Dr. Bhanu Chowdhary
- XY sex reversal, XO and XX pseudohermaphrodite
mares have been reported. XX pseudohermaphrodite mares are
phenotypically "female" yet have testes. In some cases
this has been attributed to a translocation of the testes
determining factor to another chromosome.
Early pregnancy
loss (Early Embryonic Death - EED)
- Definition: loss of the conceptus before
organogenesis is complete.
- Caution must be used in the diagnosis of
this condition.
- Diagnosis by observation of failure to return to
estrus and an assumption of pregnancy will erroneously overestimate
or possibly underestimate EED.
- Diagnosis by palpation may
underestimate the incidence of EED.
- Diagnosis by hormonal assay can
also lead to errors. A single low value of progesterone is not
necessarily indicative of impending loss. ECG will remain elevated
even after embryonic death once the endometrial cups are
established.
- Ultrasonography is the best way to detect EED. With
ultrasound, pregnancy can be confirmed and loss can be verified.
There are numerous causes of EED.
- Decreased pregnancy rates have been observed
with significant uterine pathology.
- It is hypothesized that fibrosis is associated
with uterine gland failure.
- Glandular secretions (histotrophe) are
important for support of the embryo due to delayed placental
attachment in the mare.
- If breed association regulations allow it,
embryo transfer is a treatment option for these mares.
- Progesterone supplementation has been used in
an attempt to increase pregnancy rates but results are equivocal.
-
Progesterone deficiency is often thought to be a cause of early
loss.
- However, primary luteal insufficiency has not been documented
as a cause of embryonic loss.
- Secondary luteal insufficiency, on the
other hand, is possible due to any number of causes that result in
endogenous PGF release and it is possible to rescue a pregnancy with
exogenous progestins.
- Progesterone is essential for pregnancy
maintenance. Decreased progesterone causes EED, and conversely EED
causes decreased progesterone.
- There is considerable variation in
progesterone levels between mares and between samples in a mare
during early pregnancy.
- A single low value, even <2 ng, is not
indicative of progesterone deficiency.
- Progesterone must be low for
2 consecutive days to document progesterone deficiency.
- No adverse
effects are reported from the use of exogenous P4 during pregnancy.
On the other hand, progesterone supplementation has not increased
pregnancy rates in normal mares.
- If progestin supplementation is to
be used, give adequate amounts at proper intervals.
- Many described
regimens do not significantly alter plasma P4.
- Ovariectomy at d 35
required exogenous P4 sufficient to maintain plasma levels >4 (or
at least >2) ng/ml to maintain pregnancy.
- Products and dosages
which have been shown capable of maintaining pregnancy are:
- progesterone in oil - 150 mg for the first 10-14 d then 200 mg every
other day;
- repositol progesterone - 1000 mg every 4 d;
-
BioRelease P4 LA
150
("progesterone
in biorelease
technology
vehicle") from
BET Pharm - 1500
mg, IM every 7 d
- altrenogest (Regu-Mate),
an oral progestin, - 1 ml/ 50 kg, PO, SID.
- It is generally
recommended to maintain supplementation for the first 105 -120 d of
gestation.
- If using progestin therapy, the viability of the
conceptus should be periodically reaffirmed.
- EED is associated with luteolysis or ovariectomy before
50 d of gestation.
- Between 50 -100 d of gestation, pregnancy loss is
variable after ovariectomy.
- After 120 d of gestation, ovariectomy
has no effect.
- Inappropriate release
of PGF, such as from an extra-uterine source can cause luteolysis
and pregnancy loss.
- It is possible to prevent pregnancy loss with
PGF inhibitors such as flunixin meglumine but they must be
administered within a very short time frame, i.e. they are not
practical in most clinical situations.
- Pregnancy loss does not occur
immediately after luteolysis and therefore time enough exists to
rescue the pregnancy with progestin supplementation.
- Therefore,
administration of P4 or altrenogest is more practical in most
situations.
Endometrial cysts
are not associated with pregnancy loss once pregnancy is established.
Whether they can interfere with the maternal recognition of pregnancy if
they are sufficiently large or numerous is unclear.
Failure of maternal recognition of
pregnancy:
- The embryo suppresses endometrial PGF. This is
a transient characteristic of the embryo that is observed in vitro
with d 12,13,14 embryos. This points out the importance of the
embryo-uterus synchrony that is necessary to establish pregnancy.
- Retarded development of the embryo may result
in pregnancy loss.
- Mobility of the conceptus is required to
prevent luteolysis. The embryo travels all around the uterus for
approximately 10 d after it enters the uterus, ceasing movement on
about d 16 of gestation. Experimental restriction of this movement
results in embryonic loss and the pregnancy can be rescued with
progestins.
Embryonic/oviductal defects
- It is difficult to separate defects of the
embryo or oviduct from problems caused by age of the mare.
- EED is greater for old mares and it appears
this is due, at least in part, to defective oocytes.
- Embryos from old mares are of poorer quality
than those from young mares.
- Oviductal cells from old mares have a
detrimental effect on embryonic development in vitro, compared to
cells from young mares.
- There is a significant difference in protein
production between oviductal cells of old and young mares.
- Pregnancy rates following GIFT are lower using
oocytes from old mares compared to young.
- Similarly, it is difficult to identify what
factors may be involved in "Subfertile" mares. Embryo
recovery is higher for normal than for subfertile mares.
- Subfertile mares had more abnormal embryos than
did normal mares. There was no difference in pregnancy rates at d 2
after ovulation between normal and subfertile mares, but there was a
significant difference at d 14.
- After transfer of embryos from normal mares to
normal and subfertile recipients there were equal pregnancy rates at
d 12 and d 28 in one study, but greater EED for subfertile
recipients in another study.
- After transfer of embryos from normal and
subfertile mares, collected at d 4, to normal recipients, pregnancy
rates were lower with embryos from subfertile mares.
Other causes of EED
- Nutritional stress has been associated with EED,
which could probably be prevented by supplemental feeding. Other
types of stress (hauling, weaning, etc.) have been associated with a
rise in adrenocorticotropic hormone and transient decrease in
progesterone but not with pregnancy loss.
- Foal heat has no effect on EED. Mares bred on
foal heat which are subsequently diagnosed pregnant have a normal
likelihood of carrying the foal to term.
EndometritisEndometritis
can be categorized as
- 1) sexually transmitted diseases
- 2) chronic infectious
- 3) persistent mating induced
- 4) chronic degenerative, sometimes referred to
as endometrosis. Chronic degenerative endometrosis, or periglandular
fibrosis, has been touched on already and is associated with
increased pregnancy loss.
- When we speak of
sexually transmitted endometritis, generally
we are referring to Contagious Equine
Metritis (CEM).
- This disease is due to
Taylorella
equigenitalis,
a Gram negative coccobacillus that grows slowly on chocolate agar in
5% CO2.
- It can survive for an extended period on the external
genitalia of the stallion and the vagina or clitoris of the mare.
When infected, the stallion may show no clinical signs.
- The mare,
however, shows acute endometritis with a thick, grey, mucoid
discharge after breeding and may short cycle due to the
inflammation.
- The acute signs subside rapidly with some mares
remaining as asymptomatic carriers.
- To check for the disease,
cultures are obtained from the following sites:
- in the acute state -
endometrial or cervical swab;
- in the chronic state the best site to
culture in the mare is the medial clitoral sinuses;
- in the stallion
- urethral fossa, also urethra, pre-ejaculate fraction, folds of
sheath, skin of penis. In stallions, repeated negative cultures are
needed to consider a stallion negative.
- Special transport media (Amies
with charcoal) is needed to get the sample to the lab.
- Laboratory tests in addition to culture include
serology which in most cases detects a rise in antibodies from 7-45
d post infection in mares but is of little use in detection of the
carrier
- Complement fixation which identifies the chronically
infected horse but not carriers,
- ELISA, serum agglutination which is
good for diagnosis of the acute case, passive hemagglutination, and
immunofluorescence.
- Control is important.
- This is a reportable
disease in the US with most measures aimed at quarantine to control
spread.
- It is generally regarded as not currently in the U.S.,
however CEM-like organisms were isolated two years ago in
California and Kentucky.
- Treatment:
- Topical treatment of the
stallion's penis with chlorhexidine scrub and nitrofurazone seems to
be effective.
- Most mares recover but up to 20% of mares remain
carriers in spite of treatment.
- Topical treatment of the mare
consists of IU antibiotics and flushing the clitoral fossa and
sinuses with chlorhexidine then packing them with chlorhexidine or
nitrofurazone.
- Surgical ablation of the clitoral fossa & sinuses
is used to treat the carrier mare and is required for export/import
by some countries.
- Certain isolates of Pseudomonas and Klebsiella
may be transmitted venereally.
- However, Pseudomonas and Klebsiella
may also be isolated from the external genitalia of normal
stallions.
- If these pathogens are suspected to be the result of
venereally transmitted disease, serotype the organism obtained from
the mare and the stallion and compare them.
- Dourine a venereal disease of horses, is caused
by Trypanosoma equiperdum. It is a serious disease but fortunately
not found in the US.
- Equine coital exanthema is a venereally
transmitted disease caused by a herpesvirus (EHV3). It causes
infertility as a result of reluctance to breed because of painful
lesions on the vulva, penis, and prepuce. As in other herpesvirus
diseases, persistent carriers with periodic recrudescence is common.
Chronic endometritis
- Chronic endometritis results when the barriers
to contamination of the uterus are compromised or when the uterus
that is susceptible to persistent mating induced endometritis is
subjected to repeated insults (i.e. breedings). The anatomic
barriers to contamination of the uterus are the vulva, vaginal-vestibular
sphincter and cervix. Poor perineal conformation may contribute to
the establishment of chronic endometritis.
- Etiologic agents most commonly found in cases
of chronic endometritis are Strep.
equi zooepidemicus
(the most common), E.
coli, Pseudomonas
aeruginosa,
Klebsiella
pneumonia
and yeast. Others considered pathogens in clinical endometritis
(with significant growth) include Proteus
sp., Corynebacterium
sp., Staphylococcus,
Shigella.
- In cases of fungal endometritis, Candida
albicans,
Aspergillus
sp., Mucor
sp. Are
the most commonly isolated species.
- Repeated or prolonged antibiotic treatment,
especially with a variety of antibiotics, is many times associated
with the establishment of a chronic endometritis.
Treatment
- The first step in treatment should be to
correct any anatomical defects.
- Place a Caslick's to close the
vulva;
- Perform a urethral extension to correct urine pooling;
- Repair
R-V tears; etc.
- Treatment timing
- Prebreeding
- Postbreeding
- Postovulation
- Antibiotics are often used to treat a
bacterial infection before breeding or as routine post breeding
treatments.
- Their use as routine post breeding treatments is
controversial but may depend on the timing of the treatment.
- Ideally, antibiotic use is based on sensitivity.
- Superinfections
should be avoided by using antibiotics with some discretion.
- Fungal infections are difficult to treat, at
best. Some suggested medications are:
- Clotrimazole - 500 mg daily
for 7 - 12 d;
- Amphotericin B - 200-250 mg daily for 7 d;
- Nystatin -
5X106 U for 7 - 10 d;
- dilute (1 part stock solution containing 1%
available iodine in 10-20 parts final solution) betadine. Avoid
strong iodine and chlorhexidine diacetate solutions. Chlorhexidine
is very irritating to the equine endometrium. Some mares are
sensitive to even dilute iodine, therefore
Persistent mating induced endometritis
- Normal uterine defense mechanisms are comprised
of a number of factors
- Local inflammatory response
- In response to an insult,
either bacteria or semen, the uterus mounts an inflammatory
response.
- Neutrophils, antibodies, complement, and other factors
enter the uterus.
- Both complement and antibody are required for
killing of bacteria which are then phagocytosed by neutrophils.
- Mechanical evacuation of the uterus.
- Physical removal of bacteria and inflammatory byproducts then
follows.
- All mares have a transient post breeding
endometritis which is considered physiologically normal and most
mares are able to clear the endometritis in a timely fashion.
- After
AI with fresh semen, the inflammatory reaction peaks 6-12 hr post AI
and the uterus eliminates excess sperm.
- Clearance of the
inflammatory processes must occur by 4-5 d post ovulation in order
for the embryo to survive.
- The normal mare is able to rapidly
eliminate contaminants.
- Endometritis becomes established or persists
when the natural defense mechanisms fail. Treatments should be performed only during
estrus and the mare evaluated before subsequent treatments. Mares
should be observed closely for signs of irritation.
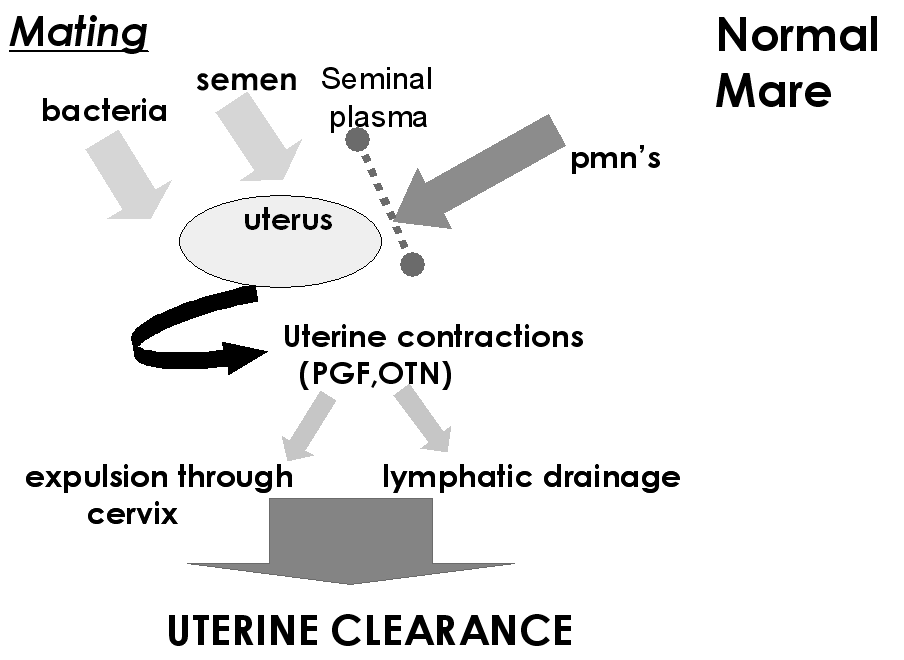
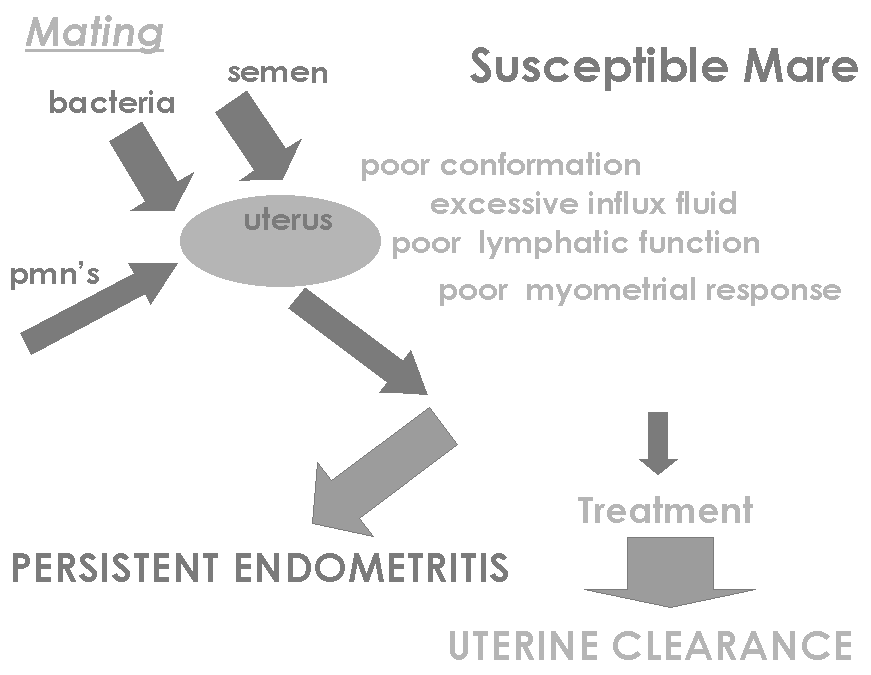
- Much effort has gone towards determining why
some mares develop a persistent endometritis after breeding while
others are resistant.
- It appears now that the primary defect is a
failure of mechanical clearance.
- Why susceptible mares have reduced
clearance is not totally clear. It is probably due to a number of
factors including reduced lymphatic function, reduced myometrial
response, conformation, etc.
- This is probably a gradual process
resulting in a loss of efficiency in bacterial clearance, although
it can occur abruptly following serious trauma.
- Studies examining uterine clearance found that
after infusion of Strep. zoo. into the uterus, resistant mares had
cleared the bacteria by 24 hr, yet susceptible mares had not cleared
it by 96 hr.
- Studies have also shown that the progesterone dominated
(e.g. post ovulation) uterus has reduced physical clearance.
- The
clearance of non-antigenic charcoal began within 2 hr in estrogen
treated mares and was completely cleared within 3 d
- Clearance
was significantly delayed in progesterone treated mares.
- Lymphatic
drainage is also impaired in susceptible mares.
- After infusion of
India ink in normal mares, ink was found in the lymphatic vessels
and lymph nodes.
- Decreased lymphatic clearance (both amount and
speed) was found in susceptible mares compared to resistant mares.
The ink remained in the lumen of susceptible mares.
- Mechanical clearance can be effectively
enhanced using oxytocin or cloprostenol.
- Uterine lavage is also very
effective in removing accumulated debris and improving the
intrauterine environment.
- Generally, when performing uterine lavage,
volumes of 1L are allowed to enter the uterus and then retrieved.
-
This is repeated until the reflux is clear.
- Lavage may be performed
as early as 4 - 6 h after breeding without reducing pregnancy rates.
- In fact, there is evidence that early lavage may be beneficial.
-
Oxytocin, 20 IU, i.v. stimulates rapid clearance within 30 min.
- Long acting oxytocin is available in Europe (
-
Cloprostenol has a longer period of action but does not stimulate as
rapid or thorough clearance as does oxytocin and may have
detrimental effects on the developing CL.
- Evidence indicates
that sexual stimulation may enhance uterine clearance by causing
endogenous oxytocin release.
- Various protocols exist such as uterine
lavage followed by 20 IU oxytocin IV, 12 hr post insemination (or
6-24 hr after each
breeding).
-
Basically, the idea is to prevent a persistent
inflammatory condition from developing.
-
Therefore, the sooner
treatment is initiated, probably the better because you are helping
to prevent a rapid increase in bacterial numbers and accumulation of
excessive fluid and inflammatory byproducts.
- Mucus or biofilm disruption
- Mucus or biofilm may protect bacteria
- DMSO - 30% solution infusion post-breeding improved conception rates
- Kerosene - 50 mL infusion - breed on next cycle
- N-acetylcysteine (NAC) -
- disrupts disulfide bonds in mucin polymers
- 3.3% solution infused not detrimental, however fertily data is not available
- Chlelating agents
- Chelates Ca or Mg to help disrupt cell wall - speculated
- Tricide - no fertilitly data yet
- Steroids
- Dexamethasone - 50 mg IV 1 hr post mating - improved conception rates (given along with other routine treatments)
- 6-12 hrs post mating (10 or 20 mg) did not improve conception (n=783 cycles)
- Prednisolone - 0.1 mg/kg q12h 4 days starting 48 hours before breeding
Diagnosis of endometritis
- Physical signs such as an exudate or evidence
of inflammation on a vaginal speculum exam are clear indications of
endometritis.
- Repeated failure to conceive after being bred to
fertile stallion is also cause for suspicion of endometritis.
-
Probably one of the best ways to diagnose persistent post mating
induced endometritis is to observe an accumulation of fluid by
ultrasound post-insemination.
- While normal mares may still have some
fluid 6 h after breeding, the fluid is gone by 12 h.
- If on
examination at 12 -24 h post mating fluid is observed in the uterus,
a diagnosis of persistent post mating endometritis can be made.
- Other means of diagnosing endometritis include
evidence of chronic inflammation on endometrial biopsy and a
positive Cytology/Culture.
- Cytology is more important than culture
in the diagnosis of endometritis.
- Neutrophils are only present in
the lumen when an active inflammation is present (need not be
septic).
- Quantitative and qualitative culture results must be
correlated with evidence of inflammation.
- Culture is used to suggest
the etiology of an already confirmed inflammatory process.
Management to prevent recurrence is as important
as treatment afterwards.
- First, reduce contamination.
- Minimize the number of breedings per estrus by
predicting when ovulation will occur. This will decrease the number
of insults to the uterus. Avoid repeated breedings during the
transitional period.
- Keep in mind that fresh sperm cells normally
survive in the mare 's oviducts for days after deposition, often up
to a week.
- The ovum, however, is fertile for only a few h
after ovulation. (Therefore when breeding with frozen semen, mares
are checked every 6 hrs and bred as soon as ovulation is detected.)
- Sperm cells are transported into the oviduct
very rapidly, therefore treatments can begin within 4 hr after
breeding.
- Use hCG or Ovuplant to induce ovulation. They
are effective in inducing ovulation of a >35
mm follicle within 24-48 h. These management techniques also reduces
overuse of the stallion.
- When possible, use AI with a semen extender
containing an antibiotic. In breeds requiring natural service, the
Minimum Contamination Technique may be used, where semen extender
containing an antibiotic is infused into the uterus before breeding.
- In mares with endometritis before breeding,
pre-breeding treatments are indicated, whereas in mares which do not
have endometritis before breeding but are prone to developing
persistent post mating endometritis, post-breeding treatments are
more effective.
- Treatments can also be used after ovulation
because the embryo does not enter the uterus for 5 days after
ovulation and the CL is not responsive to PGF until approximately 5
d after ovulation.
- Therefore, examinations after breeding should
be performed not only to check for intrauterine fluid but also to
detect ovulation.
- There is some evidence that the effectiveness
of oxytocin diminishes somewhat as progesterone rises so there may
be reason to try and accomplish uterine clearance after breeding but
before ovulation.
Endometrosis
Pyometra
- There are several possible etiologies for
pyometra in the mare.
- Cervical adhesions or malfunction with failure
to drain contents are often implicated.
- Reduced endometrial
resistance or other unknown factors may also be involved. A variety
of organisms can be involved and in some cases a negative culture
may be obtained.
- There is usually no sign of systemic illness.
- In some cases there may be a discharge.
- On rectal exam, a fluid filled uterus is found
which must be differentiated from pregnancy. The majority of
affected mares cycle normally.
- In some cases, with severe endometrial damage,
PGF release may not occur so that luteal function is prolonged. In
other cases, the mare may cycle at shortened intervals due to
endometritis and PGF release.
- In all cases, however, the prognosis for future
fertility is poor.
- The damage to the uterus is most severe in long
standing cases or those with a closed cervix.
- In these cases the
endoetrium may be replaced by granulation tissue.
- In less severe
cases endometritis with atrophy and fibrosis of the endometrium is
found. If the pyometra is cleared up, it will likely reoccur.
Cervical trauma
- These are often the result of a laceration
following a foaling injury. Adhesions may follow a dystocia with
subsequent cervicitis/vaginitis or following inappropriate therapy.
Correction requires surgery but is difficult and often unsuccessful.
Salpingitis/oviductal blockage
- Salpingitis has been noted in abattoir studies
as not uncommon, however oviductal but blockage is rare.
Endometrial atrophy
may be found on biopsy if the affected area was biopsied. Alternatively,
it may be suspected on the basis of placental inspection after
parturition.
Breeding accidents
- Whenever bleeding is observed after natural
mating, it is important to determine the origin of the hemorrhage.
It may be due to vaginal perforation. If so, the location and extent
of the tear must be determined. Vaginal tears may be peritoneal vs.
retroperitoneal depending on their location. Treatment will depend
on their location (retroperitoneal -antibiotics; peritoneal
-antibiotics, surgery or cross tie the mare).
- Prevention should be practiced if any problems
are anticipated (large stallion with small mare, stallion with
previous history). AI would certainly avoid the problem but if
natural service is required, the use of a stallion roll will improve
safety. Varicose veins in the vagina may rupture during breeding but
are not of any serious consequence.
- Rectal breeding can cause a rectal tear. If a
stallion penetrates the rectum during breeding, do a rectal exam to
check for tears. If present, treat as previously discussed.
|


 Equine
Index
Equine
Index
