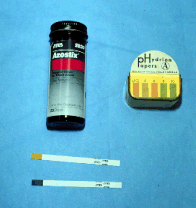Stallion
Breeding Soundness Examination
 12-15 12-15

- The goal of a stallion
breeding soundness examination is to select stallions for fertility,
eliminate stallions with heritable defects, alert owners of
subfertile stallions, and determine any cause of infertility.
- It is important to note that
the fertility is assumed for time of examination only, since
conditions may arise shortly after the examination that affect
fertility.
History
- The foaling rate is a good indication
of fertility.
- Check the foaling rate of the last
breeding season.
- Check the reproductive history of the
mares, as barren or infertile mares may make stallion look like he
has subfertility.
- Record the services/foaling, but be
careful as abortions etc. that are not associated with the stallion
may alter the number of foals born.
- Calculate the services/conception for
maiden, barren, and foaling mares.
- If a problem shows up, you may also
want to check the management (breeding and housing) of the mares to
help rule out a management problem.
- Determine the intended use of this
stallion
- Natural service vs. AI
- Fresh cooled semen?
- Frozen semen?
- Size of book (expected number of
mares to be bred
Diseases
- The stallion should be free of Equine
Infectious Anemia , Equine Viral Arteritis , CEM.
Identification
- Positive identification is essential.
 Who
am I? Who
am I?
- A tattoo is the best identification,
but a photo is also a good idea.
- In any case, make sure you positively
identify the stallion to avoid legal complications later!
General physical exam
Examine the stallion for:
- Conformation,
- Lameness,
- Vision,
- Inherited Defects,
- Cryptorchidism,
2 scrotal testes
- Combined Immunodeficiency,
- Parrot Mouth,
- Hemophilia,
- Complete Mature Cataracts,
- Aniridia,
- Wobbler,
- Multiple Exostosis.
- A breeding sound stallion should be
free from these defects.
- Ultrasound
-
Although we usually think of the mare when we consider reproductive
ultrasonography, there are a number of uses for ultrasonography in the
stallion.
- Ultrasonographic examination of the testes is an accurate
method for determining testicular size, as well as identifying
pathologic features.
- Testicular parenchyma can be examined, testicular
trauma evaluated and tumors identified.
- The central vein is an easily
identifiable landmark.
- Scrotal contents such as bowel or excessive fluid
can be visualized.
- Hematocele can be differentiated from hydrocele.
- The
internal genitalia can also be examined.
- The accessory sex glands are
better evaluated using ultrasonography than by palpation alone.
Semen collection

- Semen is collected with an artificial
vagina.
- A stallion ejaculates based on the temperature and
pressure exerted onto the penis by the vagina, or in our case, the
artificial vagina.
- Each artificial vagina is designed to produce the
correct pressure and temperature to make a stallion ejaculate.
- Each
model has advantages and disadvantages.
Artificial Vagina
Models of Artificial
Vaginas
Colorado (ARS - Animal Reproduction
Systems)
- This is a commonly used model.
It forms a water jacket using a hard shell and a rubber liner. A
second rubber liner or a disposable liner is inserted into the AV to collect the semen.
- The advantage of this system is that
it holds a large volume and will retain its heat very well in cold
climates.
- A disadvantage is that it contains a
large amount of water and is fairly heavy.
- Another disadvantage is that after
ejaculation the semen is retained in the confines of the water
liner. If the water is not drained out quickly, the sperm cells are
subjected to a deleterious temperature.
- The ARS version has an entire setup
(liner, filter, collection bottle) available which includes a disposable liner to prevent contamination
of the sample and contamination between stallions. The disposable
liner also facilitates clean up. The latex liners are reported to
cause lower sperm motility, but some stallions object to the
disposable plastic liners.
- The system also includes an in-line
semen filter to filter out the gel fraction of the ejaculate.
- If the ARS is completely filled with
50o
C water, it will equilibrate to 45o
C in about 10 minutes.
- The pressure is adjusted by opening
the valve, inserting your arm into the AV and letting water out to
the desired pressure (unfortunately this is only a guess).
- If more pressure is desired or the
temperature needs to be increased, more water must be added (this is
a pain when you are out collecting a stallion).
- Dr. Eilts' favorite.............at
least for stallion collection.
Missouri

- The Missouri AV contains the water
between two soft liners, instead of a hard shell.
- Advantages of this system include:
- You can easily adjust the pressure by
blowing air into the water bladder.
- The semen does not remain in contact
with the water after ejaculation.
- It is very light.
- Disadvantages include:
- It does not hold much water, so it
drops in temperature relatively rapidly in cold weather.
- It is difficult to get and insert
disposable liners into it, so it must be cleaned with alcohol
between each stallion. This results in a greater chance of
contamination.
- The leather case does not always hold
the AV firmly when the stallion's penis enters the AV.
-
A special "coupling nut" is needed to
attach a collection bottle to it
The Hannover AV
- Somewhat of a Nishikawa - Colorado hybrid
- Outer case is made of hard rubber
- Has a partially closed distal end for the stallion to push the end of the penis against
- Disposable liners, filters, collection bottles are available,
similar to the Colorado
- Dr. Paccamonti's favorite............for collecting stallions.

Japanese (Nishikawa)

- This is basically an aluminum
Colorado.
- It has a small hole in the cap that
is designed to vent water and automatically adjust the pressure as
the stallion's penis enters the AV.
- There is also a rubber ring in the
end of the AV that is designed for the stallion to push his penis
against and supposedly feel more natural.
- It is smaller and made of aluminum,
so it loses heat rapidly.
- The collection 'bottle' is a rubber
cone, which can be contaminated easily.
- These are no longer available.
Krakow - Polish

- This is just a short model used for
.......no not stallions that have a short penis........for
fractionating the semen sample.
- It is most useful in situations when
you want to examine the ejaculate to determine where a particular
problem originates.
See the French IMV by clicking here
AV Preparation
- Most stallions want a temperature of
45-48o
C (depends on stallion's preference).
- Adjust the pressure to stallion's
preference just before you are ready to collect him. This keeps a
lot of water in the AV and helps prevent cooling.
- Use about a tablespoon of
non-spermicidal lube when you are adjusting the pressure. Only
lubricate abut the first third of the AV. Lubricate just before
collection to avoid drying out the lube and to check for foreign
objects (thermometers) in the AV. Studies have shown that even
non-spermicidal lubricants can have detrimental effects on sperm due
to changes in osmolarity. There are some reports coming out about how spermicidal lubes are. These reports tended to use a lot of lube placed into the semen (5% by volume).
Condom
 
- Commercially available
- Hard to put on and keep on
- Have seen human condoms used on mini stallions (best
to use two)
Manual stimulation

- This can be used for stallions that
have difficulty mounting.
- You can attempt manual massage of the
erect penis with moist towels while the stallion is standing or
while he is mounting.
- Experimentally it took about 1 1/2
training sessions to train stallions to do this.
- The semen (except for pH) is the same
as if done by an AV collection.
Pharmacologic
- This can also be used for stallions
that have difficulty mounting.
- Erection and ejaculation are
primarily alpha, whereas arousal beta stimulation.
- Xylazine
- It has both alpha and beta actions.
- Use 0.3 mg/lb after sexual
stimulation.
- Experimentally semen was obtained in
about 40% of stallions.
Xylazine/imiprimine
- Imiprimine is a tricyclic
antidepressant used in humans. (It was noted that a side effect of the
drug in humans was ejacualtion)
- It is only used experimentally now.
- The 'current' method used by Sue
McDonnell and sent on the Equine List server 3/4/02
-
"We
now (2002) use the oral imipramine instead of injectable. (We
had trouble getting
injectable preparations that didn't result in some worrisome
hemolysis.)
-
For
a 1000 pound horse we now get best results starting with 1000 mg
imipramine
hydrochloride orally approximately two hours before 200 mg.
xylazine IV. Ejaculations when they occur, tend to be at about 2
minutes after xylazine or at 15-20 minutes after xylazine,
either when the horse is just becoming sedate or just regaining
alterness. We
adjust the dose for subsequent attempts
based on the horse's response.
If he goes from normally alert to fully
sedate (head down to about 8 inches from floor) very gradually
over 120 seconds after xylazine, then we consider that is a good
level of xylazine. If he goes down abruptly (less than 1 minute)
or to very deep standing sedation (wobbly swaying, snoring with
nose on floor), then too much xylazine.
If he never gets fully sedate (head down to about 6-8
inches from floor,
and not alert and responsive to surroundings) or not fully
sedate until several minutes after xylazine, then we just that
not enough xylazine.
These induced ejaculation protocols based on imipramine and
xylazine have always worked best with with a quiet stallion that
tolerates injections well.
We deliberately do the procedure at quiet times and have
a minimum of people to disturb the horse (only one). We attach a
collection bag over the prepuce on a girth strap so that after a
queit approach and injection, we can tip toe out of the stall
and leave the stallion undisturbed. we either monitor on remote
video monitor or "peak" around the corner quietly.
-
Also,
with this protocol the semen will likely be very concentrated,
with a small
volume. That's due
to the imipramine enhancing contractions of ampullae and
inhibiting somewhat the contractions of accessory sex
glands.It's great for freezing, but needs immediate careful
handling to avoid cold shock. We've had some over 1 billion per
ml. the total count
is usually higher than what you would get in an in copula
ejaculate from that horse.
When to collect
- Semen collection after sexual rest is
not indicative of how many sperm cells a stallion can produce under
normal use.
- It is best to collect a stallion when
he is at his Daily Sperm Output (DSO).
- The DSO estimates the number
of cells the testicular parenchyma can produce daily.
- This is when
all the cells he is ejaculating equals the cells he is producing
every day.
- It may take 7-10 days in some cases to get to the DSO.
- Normally the sperm output will stabilize at the
daily sperm output.
- The mean of last 3 daily collections is DSO.
- You can
estimate the DSO by taking 27.5% of second ejaculate (taken 1 hr
apart). From testicular
measurements:
- DSO=-3.36 X 109 + (0.066 X 109)(SW in
mm)
- DSO=(0.024)(TV)-0.76
. (TV=(4/3)π(0.5* height * 0.5 * width * 0.5 * length).
- It would be a good idea to collect a
stallion at his normal use schedule.
Semen Collection
- For a stallion not trained to a
dummy, an estrous mare is needed.
- If possible pick one that is calm
and does not like to kick.
- If you do not have a mare in heat , an
anestrus mare given 1 - 2 mg estradiol cypionate (ECP) will show signs
of heat.
- An ovariectomized (OVX) mare given
2 mg ECP 2-3 days in
advance is ideal, in that the temperament for the mare OVXd can be
selected, and you 'always' have a mare in 'heat'.
- A dummy or phantom
is ideal if the stallion is trained for it. Dummies do not move or
kick!
- Wrap the mare's tail and tie it off
to the side.
- Make sure the restraint is adequate
for both mare and stallion.
- Stallion preparation
- Tease the stallion to erection.
- Wash the penis with warm water only
(no soap....soap can lead to overgrowth of harmful bacteria on the
stallion's penis) !!
- Take cultures of the urethra before
and after ejaculation, and the penile shaft.
- Culture the fossa,
corona, etc. if there is a concern or a problem.
- You can expect no
abundant pathogens, and there should be only garden types (mixed
culture) seen on the cultures.
- You should not get a pure culture of
anything.
- It is advisable to pull the shoes if
at all possible to avoid injuring the mare or any of the collectors.
The semen collection

Click the movie icon to see a video of semen
collection in the stallion.

- All the handlers should be on left
side and everyone is advised to pull to the left if there is a
problem.
- The entire collection has to be a
choreographed effort by everyone involved in order to get a sample
and keep everyone safe.
- Approach the mare at an angle and
allow the stallion to mount the mare.
- Let the stallion thrust and guide or allow the
stallion to insert his penis into the AV.
- Gently touch the ventral penis and
feel the urethra for the ejaculatory pulses. Others can watch for
the flagging of the tail that indicates ejaculation.
- After the sample is collected, keep
open of the AV upright to avoid spilling the semen out.
- Immediately drain the water out to
prevent the continued exposure of the sperm cells to hot water. The
water jacket also forms a 'dam' that will prevent the semen from
draining into the collection bottle.
- Protect the semen from light and cold
shock and then begin to analyze it as soon as possible.
 Semen
analysis Semen
analysis

Stallion specifics
- The sperm cells may make large
circles due to normal abaxial midpiece, but this is normal for
stallion semen.
- If an in-line filter was not used
during collection, remove the gel with sticks or by pouring through
a filter or some gauze.
Longevity testing
- Use individual longevity tests for
motility as well as looking at fresh samples.
- Motility - immediately examine raw and extended
samples.
- Make sample consisting of a raw
(neat) sample and samples extended 1:1 and 1:4.
- Keep the samples at room temp and
prevent light or air contact.
- Estimate motility hourly until the
motility is less than 10 %.
- The goal is to have at least 10% at 6
hours.
- If the motility is < 10 % after 3
hours, the stallion generally has poor fertility.
pH

- Check the pH immediately with a pH
meter.
- Normal pH is about 7.
- A higher indicates soap
contamination, urine contamination, inflammation, or problems with
the accessory sex gland fluid.
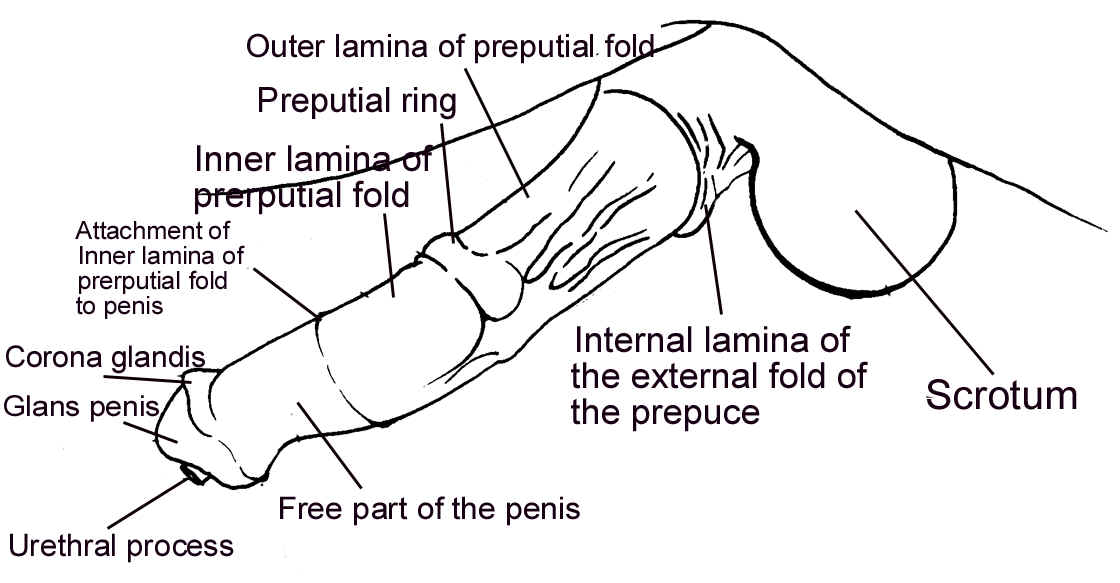
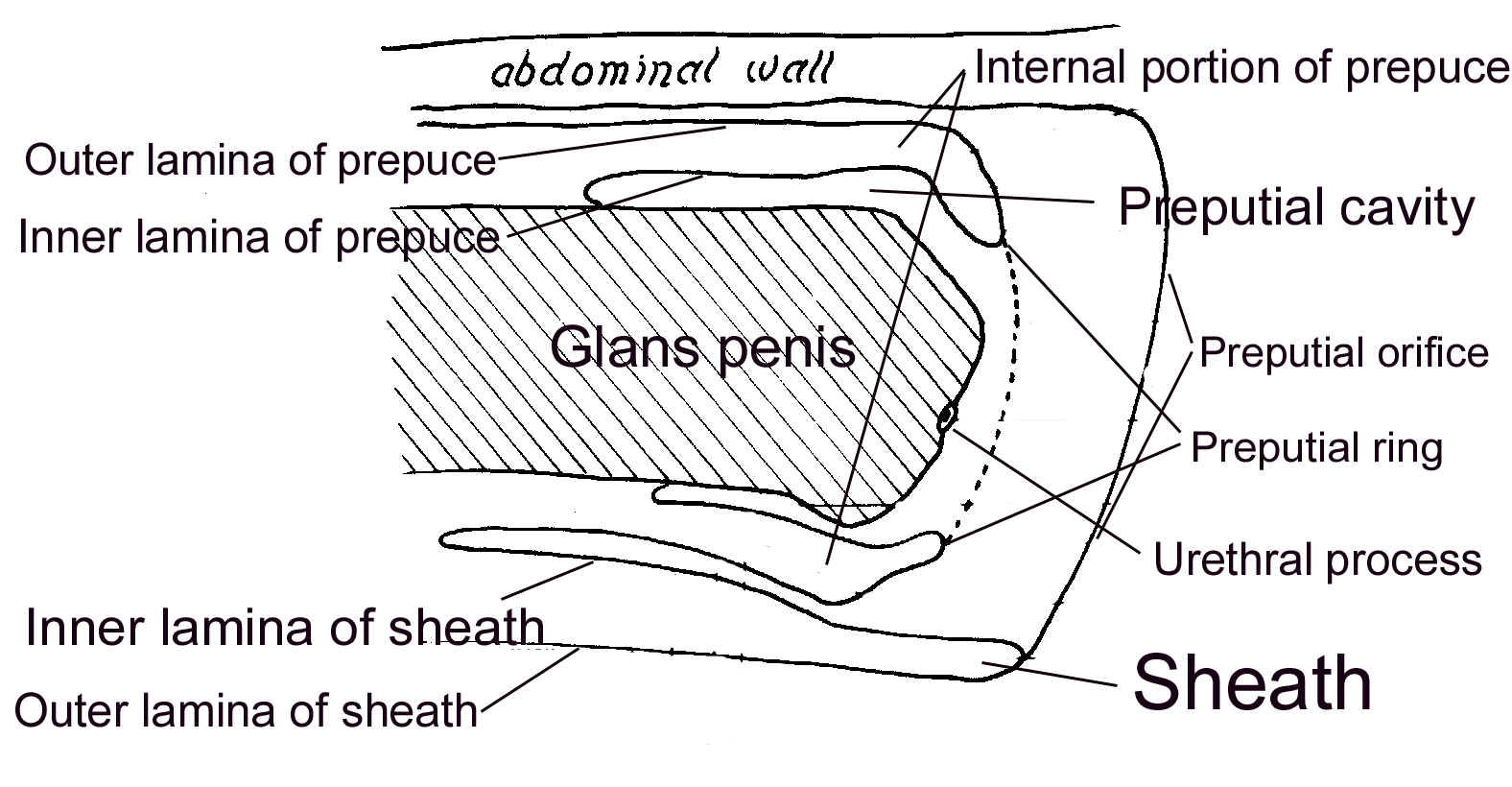
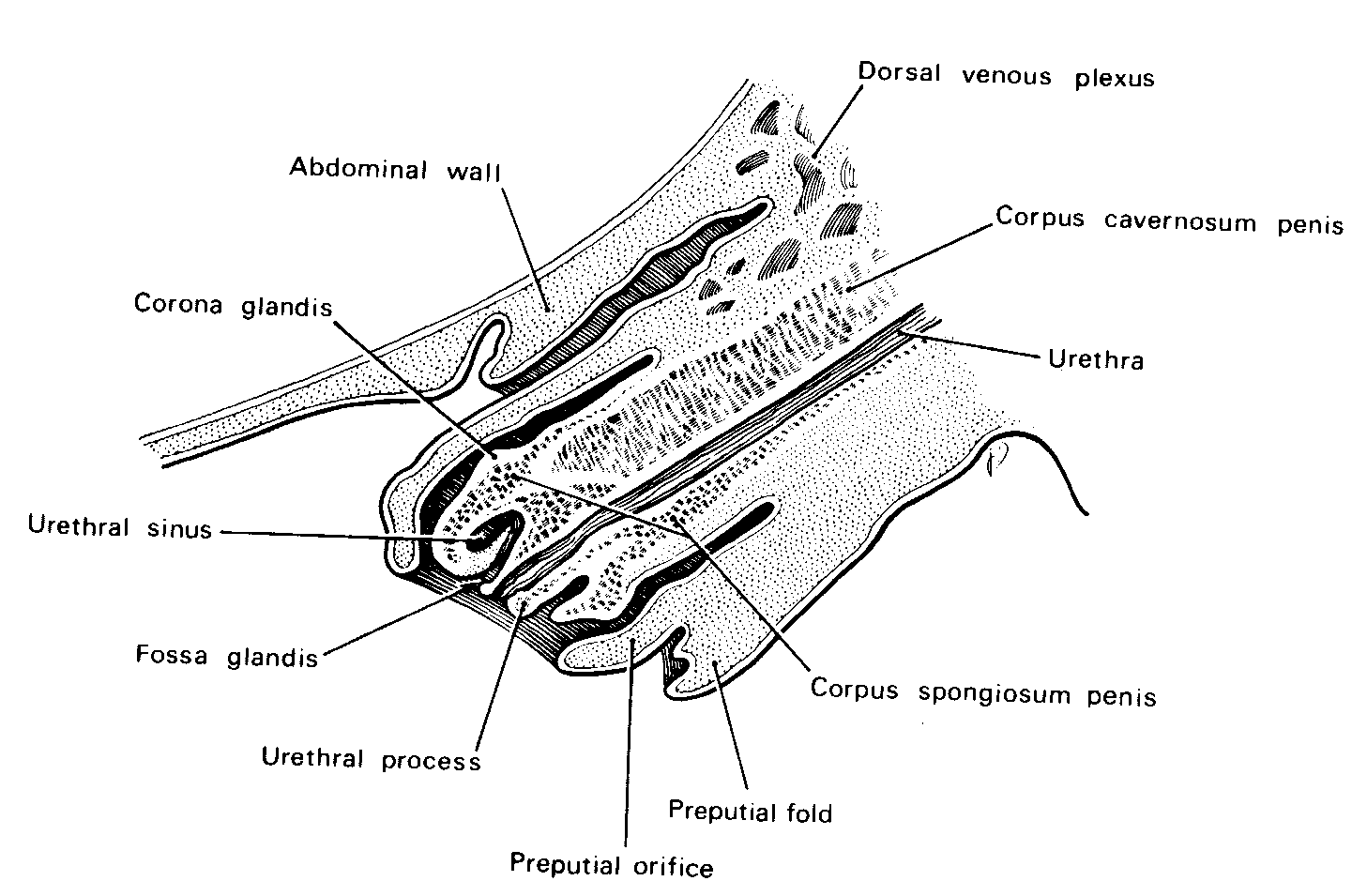
Genital exam
- A genital exam is best done after
semen collection, because the stallion will be much calmer.
- Make sure the penis is free from:
- Summer Sores (Habronemiasis).
- Sarcoid.
- Melanoma.
- Coital Exanthema (Herpes).
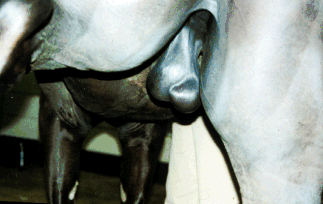
Testes and scrotum
- Check for edema of scrotum and
adhesions between the testes and scrotum that will inhibit
thermoregulation.
- Testes
- A stallion must have two!!
- Average size is 9x6x5 cm.
- The total scrotal width (TSW) should
be > 80mm.
- Ultrasound exam is best
- Measure height, width, length of testes to determine testicular volume
- Examine testicular parenchyma, epididymides
- Best way to examine scrotal contents (hydrocele, hematocele, hernia)

- The consistency should be the same
throughout.
Epididymis
- The head is anterior, the body is
dorsolateral and the tail is posterior.
- The orientation of the epididymis can
help determine if a testicular torsion exists. Transient torsions
are fairly common, and does not cause any clinical problems.
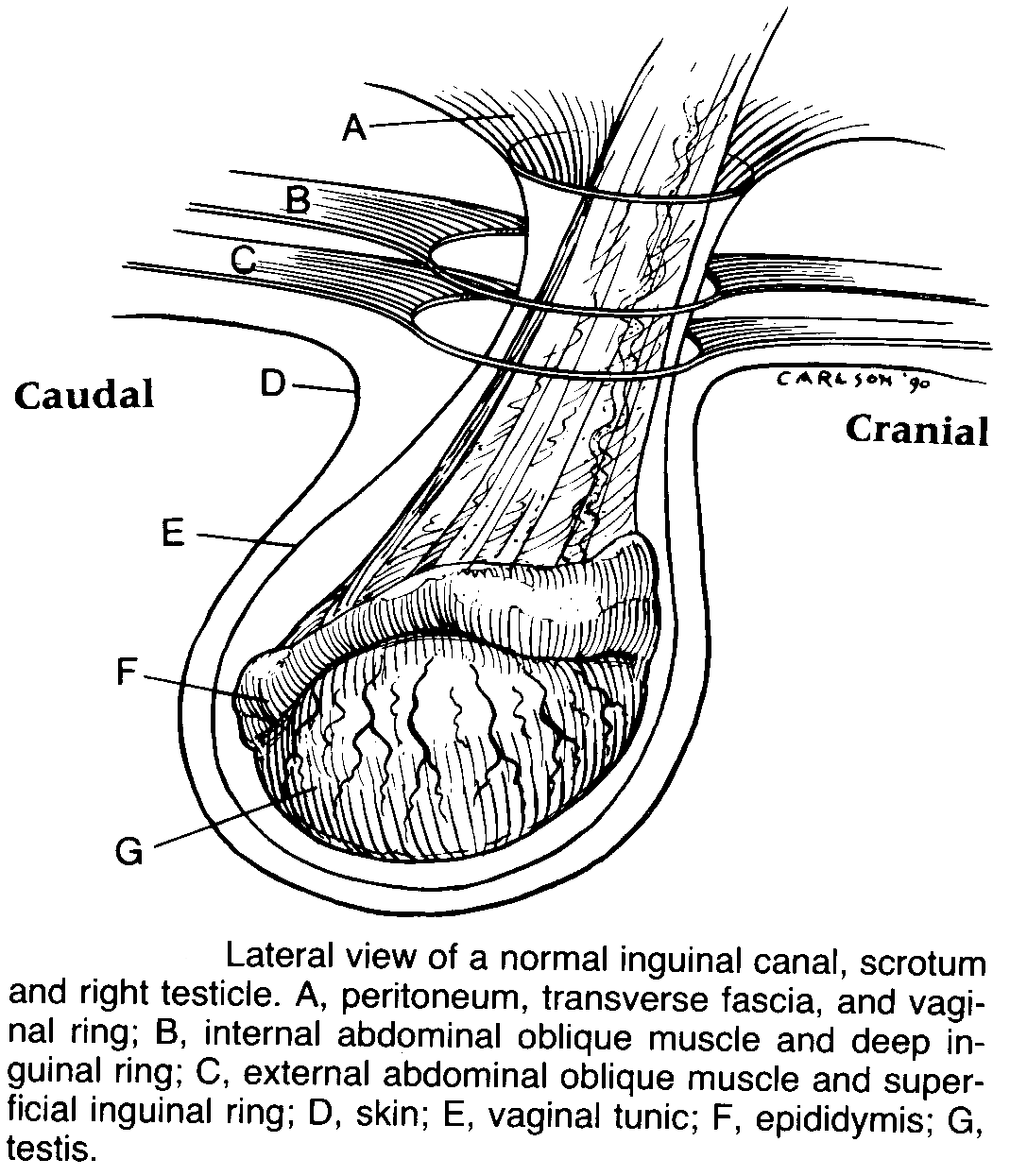
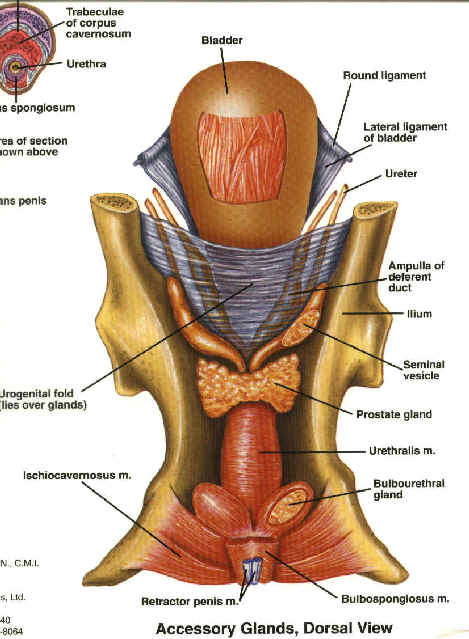
Rectal Palpation
- There are few problems diagnosed by
rectal palpation and the risk of routine exams probably does not justify the rewards.
- You can check the accessory sex
glands, which include the prostate, ampullae, and seminal vesicles.
- You can also palpate the inguinal
rings for hernias.
Second semen collection
- A second semen collection should be
performed one hour after the first collection.
- The second collection, in relation
the first, should have:
- Same Volume.
- 60 % as many cells
- Same motility
- A pH that rises slightly.
- If this relationship between the
first and second ejaculates is not seen, then one of the ejaculates
is not representative !!!
Criteria to pass BSE
- The stallion must have normal libido,
normal behavior and a normal gait.
- He should have 2 scrotal testes and
there should be no scrotal or penile abnormalities.
- The testes should have at least 80 mm
TSW.
- The cultures from the penis and
urethra should have inconsistent bacterial types, and there should
be fewer colonies after the first ejaculation. There should be no
pathogens.
- The stallion should not have
contagious equine metritis (CEM) or EIA.
- If the stallion does not pass,
recheck him again at a better time in the season or recheck him in
60 days.
Stallion
management

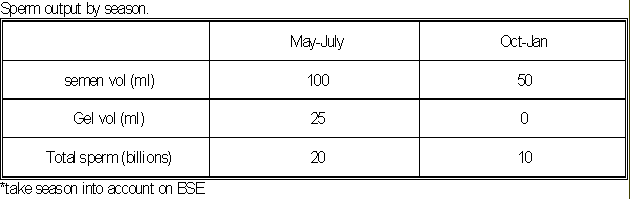
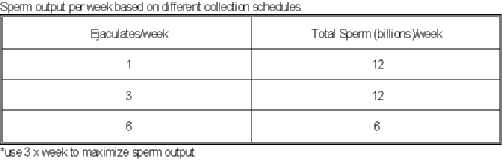
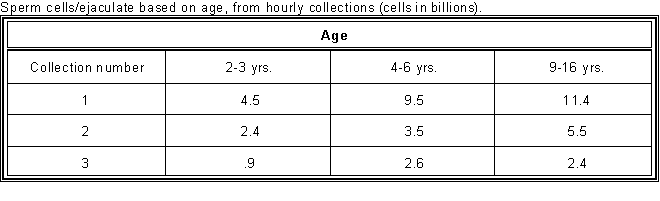
- A stallion produces sperm cells
seasonally, just as the mare cycles seasonally.
- Therefore, the season may affect the
results of the BSE.
-
PREPARATION AND SHIPMENT OF FRESH, COOLED SEMEN
- Advantages of transported fresh semen
- All the advantages of on-farm artificial insemination with fresh
semen
- Reduced chance of disease transmission
- Increased book size for stud owner
- Less chance of injury to horses and handlers
- Less expensive to ship semen than horses
- Decreased cost of broodmare care at studfarm?
- Decreased stress and chance of injury to mare and foal due to
shipping
- Breeding can continue while stallion is engaged in other
activities
- Increased availability of superior stallions or uncommon breeds
- Semen evaluation possible at time of breeding
- Disadvantages of transported fresh semen
- Lower fertility with some stallions
- Lower fertility with prolonged shipping times
- May be increased cost realized due to processing semen
- May be increased cost due to lower per cycle pregnancy
- Requires additional equipment and training for semen processing
- Requires better mare management
- Requires good stallion management
- Requires good communication between all parties
- Requires advance planning, semen may not be available every day
- Factors influencing success with transported semen
- Pregnancy rates with transported, cooled semen are similar to
those obtained after using fresh semen, provided mare management,
semen quality and semen handling are good and shipping times are
relatively short (< 24 hrs). Shipping times greater than
24 hrs are associated with some degree of reduction in pregnancy
rates, possibly as much as 50%. For these reasons, proper techniques
of semen evaluation, extending and packaging are essential.
- Semen quality / stallion fertility
- Motility, concentration, volume and morphology before extending
- Quality after storage / shipment
- Type of extender used
- Concentration, dilution ratio of extended semen
- Type of packaging system used
- Cooling rate
- Lowest temperature reached
- Ability to maintain stable temperature
- Duration of transport
- Number of normal, motile sperm inseminated
- Timing of insemination: ability to predict ovulation, use of hCG
or GnRH analogues to induce ovulation and avoid need for repeat
shipments
- Mare management / fertility
- USE OF SEMEN EXTENDERS AND SHIPMENT OF FRESH,
COOLED SEMEN
- The shipment and use of fresh cooled
semen is experiencing increasing popularity. Restrictions enforced by
the various breed associations are the main factor preventing its
widespread use. Semen extenders are an essential component of fresh
cooled semen.
- Semen extenders are an important adjunct to an artificial breeding
program. Use of semen extenders makes it possible to ship semen
overnight while preserving fertility. Their beneficial effect is
also used at times in natural breeding by infusing the extender into
the uterus in conjunction with mating.
- When an extender is added to fresh semen, it is not unusual to see
an initial increase in motility. As time passes, motility of the
extended semen remains higher and is maintained longer, than the
motility of the unextended, or raw, sample. In addition, semen
extenders containing antibiotics help to reduce the contamination
introduced into the mare's uterus at breeding. For these reasons,
semen extenders may improve the fertility.
- Semen extenders provide substances for the metabolic activity of
the spermatozoa, buffer against changes in acidity and protect
against cold shock. Glucose is the primary nutritive component of
most extenders. Depending on the particular recipe, egg yolk or milk
normally provides the protective effect against cold shock. If milk
is used, either half & half, which is heated in a double boiler
and the scum removed, or nonfat dry skim milk are usually used.
Nonfat skim milk is very easy to use, however, only brands without
added preservatives are suitable. Most commercially available
extenders rely on non-fat dry skim milk as a base. Antibiotics are
usually added to the extender to inhibit growth of bacteria in the
semen during storage. Studies indicate that the antibiotic polymyxin
B is not suitable for storage of semen, therefore it is not used in
extenders for transported semen. Although other studies have
indicated slight differences in motility after storage with various
antibiotics, from a practical standpoint antibiotics such as
ticarcillin, gentamicin or amikacin all give satisfactory results
and are commonly used.
- Osmolarity and acidity are critical factors in the preparation of
an extender. After preparing an extender, both pH and osmolarity
should be checked before use. If instruments are not available to
check pH and osmolarity, the extender should be tested to verify
that sperm viability is maintained before the extender is used for
shipping. Some antibiotics may significantly alter the pH of the
extender and sodium bicarbonate must be added to restore it to a
suitable range.
- Semen extender may be prepared in large quantities and then frozen
and stored in smaller aliquots, such as 100 ml, for later use.
Properly stored, the semen extender will be preserved for 3 to 6
months and reduce the need for frequent extender preparation.
Commercially available extenders are very easy to use and are
formulated to provide the correct pH and osmolarity. They usually
consist of a packet of dry ingredients and a small vial of diluent
which are mixed together at the time of use. Most are available in
convenient 100 to 125 ml sizes so that extender is made as needed
rather than pre-made and frozen.
- Sperm will quickly metabolize available substrates in seminal
plasma so motility will decrease rapidly. Therefore, after the semen
is collected, it should be mixed with extender as soon as possible,
preferably within 10 or 15 min. Ideally, the ratio of semen to
extender should be at least 1:2, and a ratio of 1:4 or 1:5 is
preferable. Nevertheless, it is the final concentration of sperm in
the extended sample that is the critical factor. Longevity of the
sperm cells is maximized if extender is added to give a final
concentration of sperm cells of 25 - 50 million/ml. With an adequate
extender at a proper dilution, sperm will retain their fertilizing
capability for up to 24 hours at room temperature (20oC),
depending on the individual stallion. Therefore a semen:extender
dilution factor should be calculated based on the initial
concentration of sperm to give a final concentration of 25 - 50
million sperm/ml before shipping.
- For example:
-
Concentration at collection = 267 million/ml
-
Desired concentration = 50 million/ml;
-
267 / 50 = 5.3 total parts semen + extender
-
5.3 total parts - 1 part semen = 4.3 parts
extender needed
- Therefore, use 5 parts extender : 1 part semen to give a final
extended concentration of 45 million sperm / ml
- If a stallion provides an ejaculate with a low concentration, so
that dilution at a 1:4 ratio, for example, would result in the final
concentration being less than 25 million/ml, centrifugation is
recommended. Centrifugation can be used to concentrate the semen so
that dilution at a recommended ratio can be achieved while
maintaining a concentration of 25 - 50 million/ml.
- Recommendations for centrifugation are to begin with a force of
500 X g for 10 minutes. Less time or fewer g’s will result in less
damage to the sperm from the force of centrifugation but a softer
pellet and more viable motile sperm in the supernatant that will be
discarded. Centrifugation for a longer time or at higher g’s will
achieve a better recovery and minimize sperm losses in the
supernatant but result in more damage to the sperm cells.
Centrifugation technique can be altered within a range of g’s and
times to achieve good recovery while minimizing cellular damage.
- After centrifugation, the supernatant is removed and discarded.
Studies have shown that a small portion (a minimum of 5%) of the
seminal plasma must be left with the sperm to preserve viability.
The pellet is then resuspended using sufficient extender to achieve
a final concentration of 25 - 50 million/ml.
- Preservation of semen quality depends to a large extent on the
initial quality of the semen, and varies from stallion to stallion.
- Determination of insemination dose
- Prior to shipment of semen from a stallion (and periodically
during the breeding season) it is advised to do a trial run with the
chosen shipping system and determine the motility after 24 and 48
hours of storage. This allows you to determine the "recovery
rate" and make adjustments in the number of sperm shipped so
that an adequate insemination dose will be provided when the semen
reaches its destination.
- In addition, it is advisable to maintain a record of the
collection data. Such information may help in determining the
collection or shipping frequency, shipping dose and number of doses
to expect from an average collection.
- The recommended insemination dose is usually 500 million
progressively motile (normal) sperm, although acceptable pregnancy
rates can be achieved with as few as 250 million normal,
progressively motile sperm. The volume of the inseminate is not
critical. Although pregnancy can be achieved with very small
volumes, the recommended minimum volume used for insemination is
usually 10 ml. Usual insemination doses with fresh cooled semen
range from 20 to 120 ml. Although some veterinarians are reluctant
to inseminate volumes greater than 60 ml, studies have shown no
decrease in fertility when larger volumes were inseminated, provided
the inseminate was not real dilute.
- To determine the volume of semen to package for shipment, divide
the desired number of progressively motile sperm (usually 500
million) in an insemination dose by the product of the concentration
times percent motility at the end of the storage period. For
example, if we have extended our semen to a concentration of 50
million/ml and our motility after transport is 40%, we should
package at least :
- 500 / (50 X .40) = 500 / 20 = 25 ml. Unless a stallion is in very
high demand it is best to package more than the minimum amount
needed. For example, with the above stallion, packaging 50 ml would
insure that more than adequate numbers of sperm were available for
fertilization of the oocyte. Furthermore, a more conservative method
is to include percent normal morphology into the equation. For the
stallion above, if normal morphology is 70%, the equation becomes:
- 500 / (50 X .40 X .70) = 500 / 14 = 35 ml
- Semen Packaging
- The pre-warmed extender should be added slowly to the semen. The
extended semen should be placed in a container such as a Whirl-Pak
bag, from which air can be excluded, and sealed. This container
should then be placed in a second container from which air is
again excluded and the second container also sealed.
- It has become commonplace for some people to place two
insemination doses in the shipping container, intending one to be
used upon arrival and the other to be used the following day. In
fact, there is no physiological basis for such practices. The
ideal place to store spermatozoa is in the mare’s oviducts.
Man-made semen transport devices are a means to transport semen
from the stallion to the mare without transporting horses. They
are not meant to take the place of the mare as a site of sperm
storage until fertilization. All semen received in a transport
device should be inseminated into the mare at the time of arrival.
This should be kept in mind when preparing the semen for shipment.
- Ideally, the semen should be extended and placed in the cooling
device within 10 minutes of collection. The rate at which extended
semen is cooled is critical. If the cooling rate is too fast or
too slow, sperm viability is decreased. A cooling rate of -0.05 to
-0.1 C/min is desired between 20 and 5 C. Ideal storage
temperature is 4-6 C.
- Numerous types of containers for fresh, cooled semen are
commercially available. Reusable containers such as the Equitainer
as well as inexpensive disposable systems are in use worldwide.
The systems vary in their cooling rates, minimum temperature
reached, time required to reach the minimum temperature, length of
time they are able to maintain the minimum temperature, etc.
Performance of the different systems also varies depending on
environmental conditions. Some of the disposable systems such as
the Equine Express and BioFlite compare very favorably with the
Equitainer system, while others such as the ExpectaFoal compare
less favorably, reaching temperatures below 0 C.
- Some reports indicate sperm viability is decreased due to
contact with the rubber plunger on syringes. Syringes constructed
of all plastic are therefore recommended by some researchers. In
actual practice, however, the extended semen is not in contact
with the plunger very long and the numbers of sperm cells being
inseminated are great enough that toxicity from the syringe is
probably of little clinical significance. If semen is held in
syringes for any length of time, however, as in some types of
shipping devices, syringes of all plastic should be used.
- An information form should be included with each shipment.
Minimum information required on the form includes stallion
identification, date of collection, concentration and volume of
inseminate shipped (i.e. numbers of sperm), initial motility, type
of extender used, numbers of doses shipped and any special
instructions.
- Prior to shipment of semen from a stallion (and periodically
during the breeding season) it is advised to do a trial run with
either shipping system and determine the motility after 24 and 48
hours of storage. This allows you to make adjustments in the
number of sperm shipped so that 500 million motile sperm will be
provided when the semen reaches its destination.
- Use of some of the more common packaging systems are described
in more detail:
EQUITAINER

- The Equitainer system is a very durable, hard plastic
insulated container. In the center of the Equitainer is a
well. The system is designed to cool a volume of 120 to 170
ml of fluid at the optimum rate. The extended semen sample
is nestled in ballast bags in a plastic cup so that the
total volume of the semen plus the ballast bags is between
120 to 170 ml. Each ballast bag holds 60 ml of a colored
fluid. Ballast bags should be warmed in an incubator (37oC)
for 4 hours before use. The plastic cup containing the semen
is placed into the "isothermalizer". One or two
(depending on the model of Equitainer) specially designed
freezer packs are placed in a plastic bag and loaded into
the well of the Equitainer. The isothermalizer is then
loaded into the well on top of the freezer packs. Records
should be enclosed that identify the source of the semen and
for the person breeding the mare to fill out. The particular
forms may differ depending on the breed involved. The
container is then closed and latched. It is a good idea to
seal the container so that it will be evident if any
tampering occurs.
- A sample of the extended semen that was packaged in the
Equitainer should be kept in a similar manner for evaluation
24 hours later, as a quality control. If a second Equitainer
is not available, the cooling and storage procedure can be
simulated by placing a sample in a Whirl- Pak bag which is
then placed in a polypropylene cup. The cup is then placed
in a water bath of approximately a pint of water in a
container about 6 inches in diameter, which has been
prewarmed to 37oC. Place the whole assembly into
a refrigerator set at 5oC. After a 24 hour
interval, warm the semen to 37oC and assess
motility.
- Various models of the Equitainer system are available.
Models differ somewhat in the length of time they will
maintain the semen chilled and whether a lead shield is
present. It should be remembered, though, that shorter
storage times result in improved fertility.
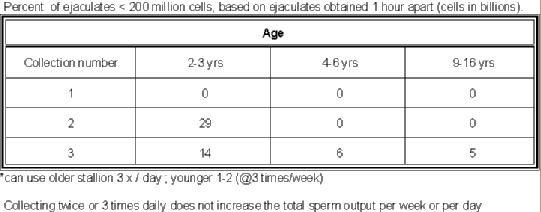
- EQUINE EXPRESS

- A number of "disposable" semen shippers are currently
available, one of which is the Equine Express. It is inexpensive and consists of a cardboard box with styrofoam inserts and compartments for the semen and a freezer
pack. The semen is packaged in all-plastic syringes. After
collection, evaluation and extension of the semen, the
extended semen is placed in an all plastic syringe. The
syringe containing the extended semen is placed in the
styrofoam box along with another syringe containing an equal
volume of water as "ballast’ to moderate the cooling
rate. Alternatively, the semen can be packaged in 2 syringes
of equal volume. A styrofoam sheet is then placed in the box
over the syringes and a specially formed freezer pack placed
on top of that. Breeding forms and other pertinent information
should be enclosed before sealing the box.
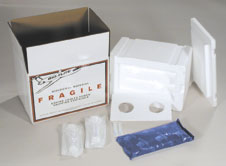
- Another inexpensive yet dependable disposable shipper is the
BioFlite. It is similar in some ways to the Equine Express, in
that it consists of a styrofoam box with a lower compartment to
hold the semen, an upper compartment that holds a freezer pack
and a styrofoam sheet between the compartments. In previous
models the semen was packed in plastic bags that were then
placed in a plastic specimen cup. In newer models of the
BioFlite the semen is packaged in all-plastic syringes.
BioFllite also makes a model for shipment of canine semen.
Made by Plastilite, Inc. Either a cardboard or plastic outer
shell is available, and either a styrofoam or special insulating
linerr are available.
- Transport and Insemination
- After packaging, the container is shipped by commercial carrier
or airline to be delivered within 24 hours. Some concerns have
been raised about x-radiation of semen as it passes through
airport security. Studies examining the effects of doses of
radiation used in airport security have found no adverse effects
on spermatozoa. However, there are indications that the airports
will soon increase the level of radiation in an attempt to improve
security and the effect of the increased level of radiation is
unknown. The Equitainer system has a lead shield in the transport
container to shield the semen from the radiation and any possible
harmful effects.
- When the semen arrives at its destination, the mare is prepared
for artificial insemination. Either while the mare is being
prepared or after she is inseminated, the semen should be examined
to determine percent motility. Care must be taken to maintain the
semen at the chilled temperature in the container until ready to
place it into the mare. The best place to rewarm the semen is in
the mare's uterus. Prewarming the semen before placing it into the
mare decreases conception rate. A drop may be removed from the
sample container and placed on a warm microscope slide on a slide
warmer. A warm cover slip is placed on top and motility estimated
in the same manner as during a breeding soundness examination.
Motility will improve as the sample is allowed to warm. The
concentration may also be determined if it is unclear how many
intended insemination doses were sent.
- Shipping
- The use of fresh, cooled semen provides a number of advantages.
It is much easier to ship a container of semen to a mare than to
ship a mare and foal to a stallion. Not only is cost
decreased by shipping semen
rather than horses, but stress on the horses is greatly reduced
also. Fresh, cooled semen allows more efficient use of a stallion,
not only for shipping, but for temporary storage on the farm to
reduce the frequency of collection. For example, a stallion can be
collected, the ejaculate extended and a portion used to inseminate
mares that day. The remainder can be cooled and used to inseminate
mares 24 to 48 hours later. Shipment of semen increases the
availability of superior stallions or stallions of uncommon breeds
and allows a stallion to breed a greater number of mares in a
season.
- Some slight disadvantages are inherent in the use of shipped,
fresh cooled semen. For unknown reasons, considerable variation
exists between stallions in the ability of their sperm to remain
viable during the cooling and storage process. For all stallions,
however, fertility is generally higher if the storage period is
shorter. If care is taken in the preparation of fresh, cooled
semen and mares are managed well, pregnancy rates using fresh,
cooled semen can be as high as those with a natural breeding
program. An important consideration if semen is being shipped in
to breed a mare is the advanced planning required. This may be
complicated by the fact that semen may not be available every day
of the week due to the work schedule of the shipping company. In
addition, use of shipped semen requires good mare management. Good
record keeping and a good teasing program are integral components
of a successful breeding program. Ability to predict ovulation is
essential in order that semen arrive before the mare ovulates, and
to avoid repeated shipments of semen during a single estrus.
Research has shown that breeding too long after ovulation results in
decreased pregnancy rates and increased early embryonic death.
Stallion
Infertility
 16-23 16-23
Management
- Check the mares to make sure
that the mares are not the source of the problem.
- Overuse may lead to oligospermia, so
it is important to check sperm output at normal use levels.
- If the stallion is being overused,
you may need to decrease the stallion's use to get greater numbers
of sperm in the ejaculate and improve fertility.
Behavioral Problems (Click on
the logo to visit the U Penn Web site on behavior)

- Often times sexual behavior is
induced by punishing normal sexual behavior.
- It may take much retraining to change
this sexual behavior.
Low Sperm Motility

- Abnormal cells may result in low
motility. There is not an immediate cure for this.
- A problem with the accessory sex
gland fluid may decrease sperm cell motility.
- You can extend the
semen to see if diluting the seminal fluid will negate the effects
of the accessory gland fluid.
- You may need to remove almost all the
accessory gland fluid by centrifugation (900 g for 20 minutes does
not normally harm the sperm cells).
Sperm Stasis
- This may be a storage problem in the
ampullae.
- You need to increase the frequency of
collection to improve motility.
- Treatment options: ampullary
massage followed by daily collection; administration of oxytocin or
PGF
Anti-sperm antibodies
- Anti-sperm antibodies, as a result of
testicular trauma, can cause low sperm cell motility.
- Corticosteroids have helped in this
condition.
Testicular degeneration
-
There are
numerous causes for testicular degeneration. Although not
clearly documented as it has been in other species, age related
testicular degeneration probably occurs in stallions. A
diagnosis of testicular degeneration can be made on the basis of
history, physical examination and semen evaluation.
Observations on physical exam include somewhat soft testicles
initially with a noticeable change in size being evident later
on in the course of the disease. Without frequent or sequential
examinations, however, slight changes in consistency or size are
difficult to discern. Findings such as a relatively large
epididymis may indicate that the testicle has decreased in
size.
-
In many cases,
only one testis is initially affected and the other eventually
follows, leading to the belief that degeneration in one testicle
affects the other testis, leading to degeneration in it as
well. Examination of the ejaculate often reveals a low
concentration of sperm, low (<20-30%) motility and
increased numbers (40-60%) of abnormal sperm resulting in a low
number of morphologically normal, motile spermatozoa.
-
Hormone testing
presents a challenge. GnRH cannot be measured directly in the
peripheral circulation. Therefore, concentrations of
testosterone and LH are used to infer the release of GnRH.
Because of the pulsatile release of GnRH and the variation by
time of day, the concentrations of testosterone, LH and FSH are
not constant but vary greatly. Therefore, several blood samples
must be collected at frequent intervals over the course of a
day. In addition, concentrations which are apparently adequate
for normal function vary between individuals and measurement of
the gonadotropins varies greatly between laboratories.
Seasonal variation also complicates the picture. Nonetheless,
hormone testing is usually recommended in order to establish a
baseline with which to compare the effects of treatment. GnRH
challenge tests are sometimes used to evaluate the pituitary
response to the hypothalamus and, provided that is normal, the
subsequent response of the testis. An hCG challenge test
bypasses the hypothalamic-pituitary portion of the axis and
assesses testicular response to gonadotropin stimulation.
Subfertile stallions respond to challenge tests with little or
no response. In the early stages of degeneration, hormonal
imbalances may not be evident but with time an increase in FSH
and a decrease in estrogen and inhibin will be observed. When
sperm concentration begins to decline, a decrease in
testosterone will also be evident.
- Roser's
work suggests the problem is at the level of the testis, not at the
hypothalamic-pituitary axis. If this is so, GnRH therapy would be
expected to be of little benefit. Evidence supports the existence
of a paracrine/autocrine system in the testis which is important for
normal function. Roser proposes that idiopathic infertility begins
at the level of the testis with an initial decline in important
testicular factors necessary for interactions between the Sertoli
and germ cells. Subsequently, a decrease in inhibin is observed,
accompanied by abnormal sperm production, an increase in FSH
release, Leydig cell dysfunction, a decrease in testosterone
production and an increase in LH.
Urospermia (urine in the semen)
- This happens when the stallions
urinates as ejaculation occurs.
- The pH of the ejaculate is higher
than normal.
- Oftentimes you can smell the urine,
but you can also use an Azostix and see if it turns green at 10
seconds.
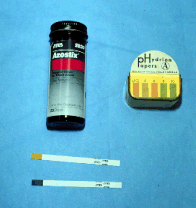
- The increased osmolarity is the
primary cause of the low motility.
Treatment
- Have the stallion urinate before
ejaculation (training).
- 'Urospas'
is a drug used in humans to increase the sphincter tone.
- You may try imipramine or bethanocol
to prevent urination during ejaculation.
Hemospermia
- It is the RBC component, not the
plasma that is harmful to the sperm cells.
- The cause is usually trauma or
overuse.
- The result is granulation tissue in
urethra. A secondary result is bacterial urethritis and seminal
vesiculitis.
- The blood may be may be from an
exterior blood contaminant also.
Diagnosis
- Collect semen in AV and look to see
if blood is in it.
- Fractionate the ejaculate to find the
source of the blood.
- Presperm is bulbourethral, sperm rich
is the prostate and ampulla (high in ergothionine), post sperm is
the seminal vesicles (high in citric acid) and the tail end is the
ampulla and seminal vesicles.
- A fiberoptic exam of urethra may
reveal ulcers, but you may not see any until the penis is erect. You may
see clots at the ducts where the vesicles enter the urethra. The bulbourethral glands have multiple ducts on the midline, the
ejaculatory ducts (ampulla and seminal vesicles) are paired and are
dorsal to the bulbourethral ducts, the prostatic ducts are lateral
to the ejaculatory ducts.
- Radiography using barium contrast may
reveal erosions and pits.
Treatment
- Determine the cause before treating.
- Sexual rest may solve the problem.
- Antibiotics such as pessaries placed
in the urethra may be needed.
- A urethrotomy sometimes allows the
erosions in the penis to heal.
- clotting agents such as Vit K have
been recommended.
- Urinary acidifiers have also been
recommended.
- Surgical treatment of strictures
caused by stallion rings may be a sequel.
- Do not cauterize
the lesions. This will result in stricture formation.
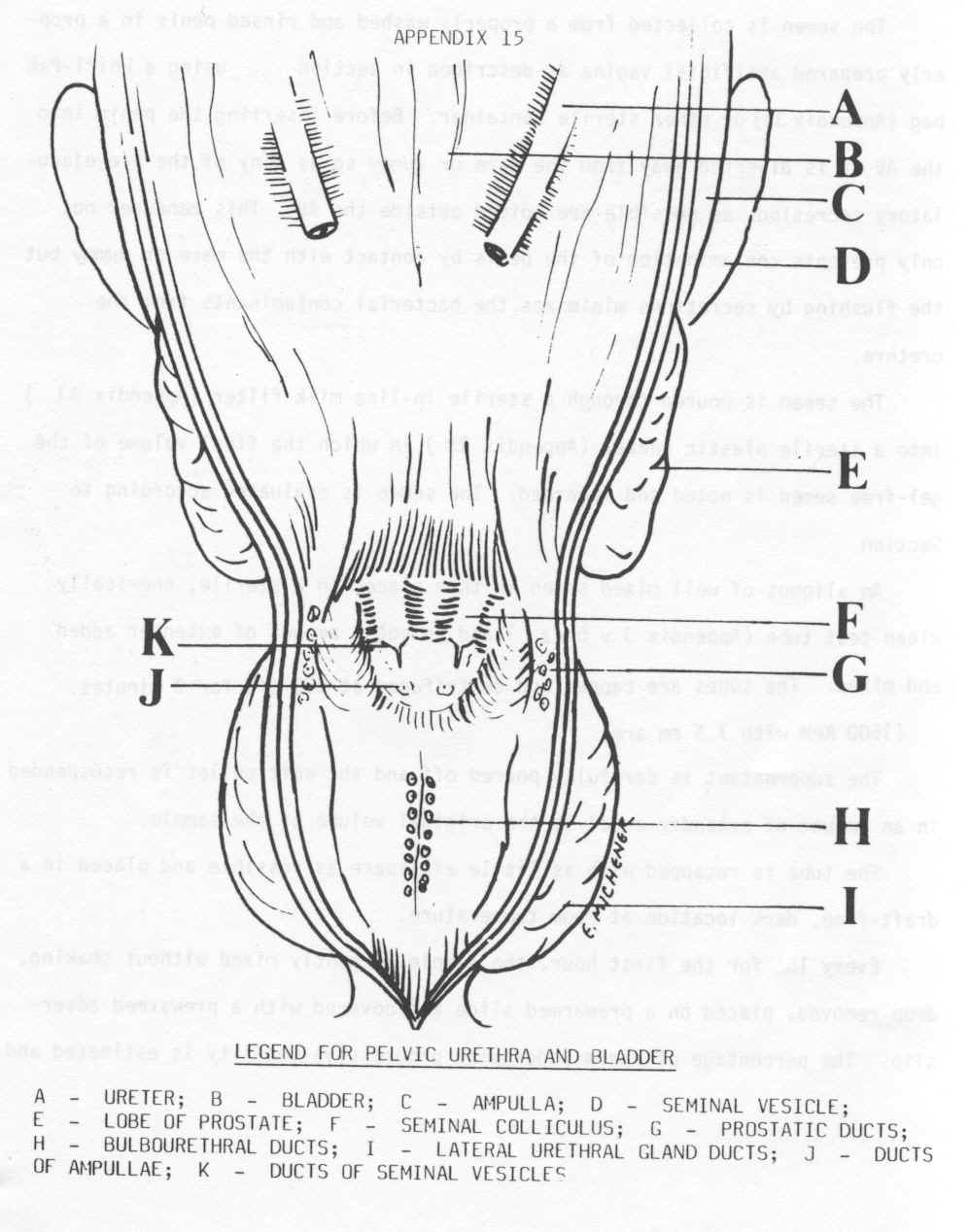
View of the duct entrances when doing endoscopy of the penis.
Shy breeders
- 0.02 mg/lb Valium 10 minutes before
breeding may allow a shy breeder to breed more efficiently.
Venereal diseases

Contagious
Equine Metritis (CEM)
- Caused by Taylorella
equigenitalis
- Signs are usually in mares, not
stallions.
Diagnosis
- Culture
- Complement fixation is good for
carrier state. It takes 10 days to become positive.
- Serum agglutination test is very good
and there are no false positives or negatives.
- It is a reportable disease
Treatment
- Topical 4% chlorhexadine scrub
followed by nitrofurazone dressing for days.
Control
Pseudomonas,
Klebsiella, E. coli
- Seen when normal commensals are
destroyed.
- Must subtype and match to the
organism grown in them.
- These bugs are almost always on the
sheath and not from the accessory sex glands.
- The treatment is using a minimal
contamination breeding technique.
- Here is a nice link to an Acrobat file from the UK standard of practice (If the link does not work you can cut and paste it into your browser:
www.hblb.org.uk/sndFile.php?fileID=%203
Equine
viral arteritis (EVA)
- Shed in semen post infection for
years after infection
Control
- Shedding stallion
- Notification of mare owners of
shedding
- Only seropositive mares may be bred
- Mares vaccinated > 21 days
or mares previously exposed to virus
- Sero-positive stallions
- Must differentiate vaccinated stallions from carriers
- Documented seronegative before vaccination
- Virus isolation on semen
Vaccination-
controlled by individual States
Coital
Exanthema
- Caused by herpes 3 virus.
- Vesicles form on the penis and
eventually form ulcers.
- There is no transmission after the
ulcers heal.
- Treatment
- Sexual rest for 3 weeks.
- Topical antibiotics.
- Control: Do not breed mares or
stallions with active lesions
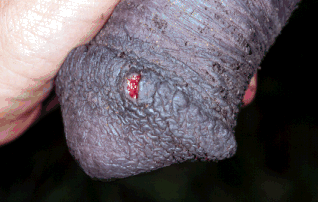 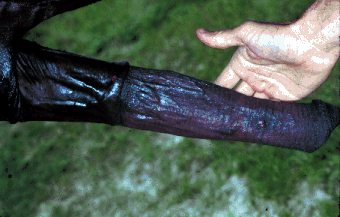
Dourine
- Trypanosoma equiperdum
- Signs
- A mucopurulent urethral discharge.
- Penile paralysis
- 50-70% mortality
- Diagnosis is isolation of the
organism.
- This is not in the United States.
Testicular degeneration
Other Stallion problems
Testicular / penile trauma
- Kicked by mare during breeding
- Swelling, edema, and possible
hematoma formation.
- You must reduce the swelling
and inflammation ASAP by using hydrotherapy and anti-inflammatories.
Frequent short applications of hydrotherapy and cold packs are
preferable to infrequent long periods. Don't overdo the cold
packs because you can get a rebound inflammation from making
the scrotum too cold ("frostbite syndrome").
- Do not use invasive measures
unless, you are going to perform a unilateral castration.
Unilateral castration may be advised in some cases, because
degeneration of one testis often affects the other testis,
plus damage to one may result in production of anti-sperm
antibodies.
- Cryptorchidism
- Penile Paralysis
- Penile Hematoma
- Penile Sarcoids
- Penile Habronemiasis:
treatment / prevention - ivermectins
- Squamous Cell Carcinoma of Penis
- Testicular Torsion
- May be acute or chronic
- Acute is usually accompanied by signs of abdominal pain (colic)
- Chronic may be intermittent and without clinical signs or may cause intermittent discomfort during sexual activity and reluctance to ejaculate
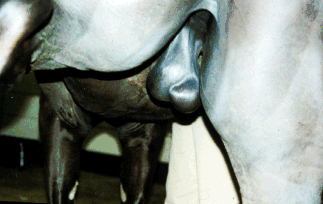
Abnormal Position of the epididymis.
(Rear view with the left rear leg
adducted) |
 Who
am I?
Who
am I?


















 Male
Index
Male
Index